Europe Blood Plasma Derivatives Market
No. of Pages: 158 | Report Code: BMIRE00028468 | Category: Life Sciences
No. of Pages: 158 | Report Code: BMIRE00028468 | Category: Life Sciences
The elderly population is rising across the world. Three groups of hematological neoplastic disorders, including multiple myeloma, acute myeloid leukemia, and chronic lymphatic leukemia, increase with age. Multiple myeloma is cancer related to blood plasma and mainly affects the elderly. As per the Global Ageing 2019 survey, the population of people aged 65 and above across the world was 703 million in 2019. Furthermore, the elderly population is expected to double to 1.5 billion by 2050. According to the WHO, the percentage of people aged 60 and above is estimated to reach 22% by 2050 from 12% in 2015. Furthermore, the United Nations World Population Ageing 2017 report states 962 million people worldwide were aged 60 or above in 2017, and the number is expected to reach ~2.1 billion by 2050. Moreover, according to Eurostat, there were 101.1 million elderly in the EU-28 at the start of 2018, accounting for about one-fifth (19.7%) of the overall population. The number of older people in the European Union (EU) is expected to rise steadily over the next three decades, culminating at 149.2 million by 2050; their relative share of the total population is expected to increase gradually, reaching 28.5 % by 2050.
One of the most recognized consequences of aging is a weak immune system. For people aged 65 and above, plasma therapy is the most successful therapy to treat multiple physiological disorders. Aging can cause changes in the blood marrow. Thus, there is an increased risk of developing cardiovascular, neurological, and blood-related disorders. Therefore, the rise in the prevalence of such disorders among geriatric populations is fueling the growth of the blood plasma derivatives market.
The Europe blood plasma derivatives market has been segmented into Germany, France, Italy, Spain, the United Kingdom, and Rest of Europe. Germany is expected to account for the largest market share and is expected to develop moderately in Europe. Europe is the second-largest market for blood plasma derivatives. As indicated by the diary of BMC Infectious Disease, in November 2021, it was accounted for that the healing plasma treatment is utilized for the patient contaminated with COVID-19. The Meta - investigation clinical preliminary led to breaking down the proficiency and adequacy of recuperating plasma. The outcome shows a decline in the death rate in COVID-19 patients by gaining strength in plasma treatment. Additionally, the flood in the number of R&D exercises and the rising government subsidizing will add to the territorial development. For instance, in August 2020, Kedrion Biopharma (Italy) began improving a plasma-inferred treatment for treating COVID-19 infection that could make it accessible to patients in just three to a half year. In May 2020, the European Blood Alliance (EBA) combined efforts with the European Commission, comprising DG SANTE, DG CNECT, and DG DIGIT. This association assisted with working and dealing with an EU-wide, open-access stage that assembles information on the COVID-19 gaining strength plasma treatment.
Furthermore, as per World Health Organization (WHO), out of 250,000 individuals diagnosed with PID (primary immune deficiencies) in the US in 2017, roughly 125,000 get month-to-month mixtures of immunoglobulins. It is assessed that more than 300,000 patients get monthly-to-month immunoglobulins implantations for PID. The rise in chronic conditions and increasing demand for immunoglobulin drive the development of the blood plasma derivatives market.
Strategic insights for the Europe Blood Plasma Derivatives provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Europe Blood Plasma Derivatives refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.Europe Blood Plasma Derivatives Strategic Insights
Europe Blood Plasma Derivatives Report Scope
Report Attribute
Details
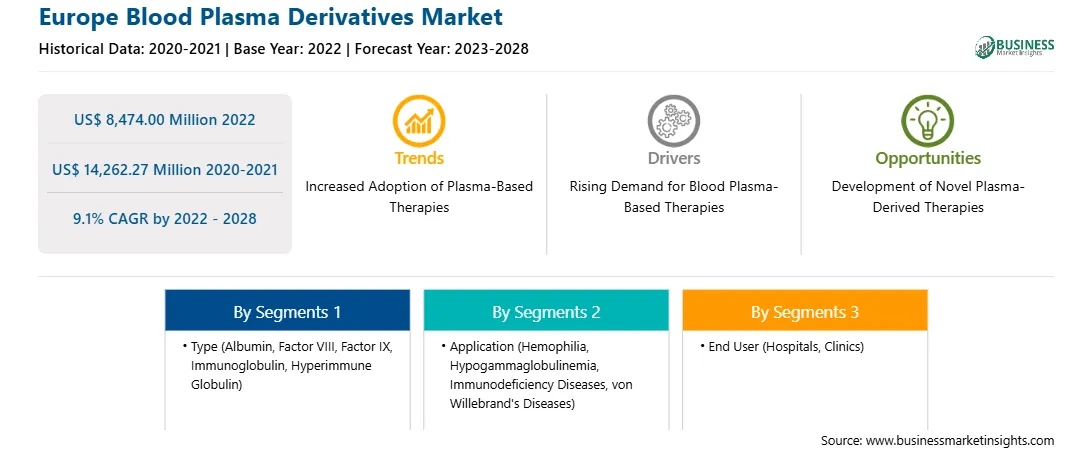
Market size in 2022
US$ 8,474.00 Million
Market Size by 2028
US$ 14,262.27 Million
Global CAGR (2022 - 2028)
9.1%
Historical Data
2020-2021
Forecast period
2023-2028
Segments Covered
By Type
By Application
By End User
Regions and Countries Covered
Europe
Market leaders and key company profiles
Europe Blood Plasma Derivatives Regional Insights
The Europe blood plasma derivatives market is segmented into type, application, end user, and country.
The Europe blood plasma derivatives market, by type, is segmented into albumin, factor VIII, factor IX, immunoglobulin, hyperimmune globulin, and others. The immunoglobulins segment held a larger market share in 2022.
Based on application, the Europe blood plasma derivatives market is divided into hemophilia, hypogammaglobulinemia, immunodeficiency diseases, von Willebrand disease, and other applications. The immunodeficiency diseases applications segment held the largest share of the market in 2022.
The blood plasma derivatives market, by end user, is segmented into hospitals, diagnostic laboratories, clinics, and other end users. The hospitals segment held the largest share of the market in 2022.
Based on country, the Europe blood plasma derivatives market is segmented into Germany, France, UK, Italy, Spain, and the Rest of Europe. Germany dominated the market in 2022.
Grifols SA; SK Plasma Co Ltd; Octapharma AG; Monobind Inc.; Intas Pharmaceuticals Ltd; Takeda Pharmaceutical Co Ltd; CSL Behring LLC; LFB SA; Kedrion SpA; and Fusion Health Care Pvt Ltd are the leading companies operating in the Europe blood plasma derivatives market.
The Europe Blood Plasma Derivatives Market is valued at US$ 8,474.00 Million in 2022, it is projected to reach US$ 14,262.27 Million by 2028.
As per our report Europe Blood Plasma Derivatives Market, the market size is valued at US$ 8,474.00 Million in 2022, projecting it to reach US$ 14,262.27 Million by 2028. This translates to a CAGR of approximately 9.1% during the forecast period.
The Europe Blood Plasma Derivatives Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Blood Plasma Derivatives Market report:
The Europe Blood Plasma Derivatives Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Blood Plasma Derivatives Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Blood Plasma Derivatives Market value chain can benefit from the information contained in a comprehensive market report.