The Europe arrhythmia monitoring devices market includes the consolidated markets for France, Germany, United Kingdom, Spain, Italy, and Rest of Europe. Germany is one of the significantly developed economy in the Europe. The healthcare expenditure is increasing in Germany. Germany's spending on health care is high as compared to the ones in other country just over 11% of its wealth, the country houses many geriatric patients suffering from chronic diseases due to various factors. The increasing prevalence of cardiovascular diseases in Germany influences the growth of the arrhythmia monitoring devices market. Cardiovascular disease continues to be the most common cause of death, i.e., accounting 40% of death. Also, since decades, it is responsible for 43.9% of deaths in women and 36.1% in men. Moreover, in Germany, CVD contributes to the largest share of treatment costs. Additionally, it is associated with significant health consequences for the individual and high medical costs for society. Due to its high prevalence, coronary heart disease, heart attacks, and stroke have exceptionally high public health relevance. Elderly people are particularly affected, but the number of sufferers under the age of 50 is rising. According to the Deutsche Welle (DW), a German public state-owned international broadcaster, the people suffering with cardiovascular conditions at present is one in five Germans over the 65 ages, and by 2060, it's expected to be one in three. Moreover, according to S&P Global Ratings, Germany's aging and declining population could increasingly burden public finances and drag on credit quality over the coming decades.
The European economy is severely affected due to the exponential growth of COVID-19 cases in the region. Spain, Italy, Germany, France, and the UK are among the most affected European countries. As per the Worldometer, as of 23 June 2021, Spain, UK, Italy, Germany, and France recorded 4,651,988; 4,254,294; 3,731,287; and 5,760,002, COVID-19 cases respectively, and the deaths toll is also high in these countries. The current coronavirus (COVID-19) outbreak has been declared by WHO as a public health emergency of international concern according to the International Health Regulation. Also, the pandemic has halted the adoption of new medical device policy and delayed clinical trials and disrupted processes. Two consecutive waves of coronavirus had devastating impact on economic activities in the Europe. Implementation of social distancing and lockdown policies led to closing of cardiac centers and patient visits for elective heart treatments. This factor negatively impacted the adoption of arrhythmia monitoring devices in the region. The European Commission recently postponed the application date of the MDR for one-year due to COVID-19 pandemic. The Medical Device Regulation (MDR) requires manufacturers to conduct Post Market Clinical Follow-Up (PMCF) studies to demonstrate the continued safety and performance of their devices. This is expected to impact the new product launches and ongoing clinical trials of medical devices, impacting sales and growth of the Europe arrhythmia monitoring devices market. In addition, disrupted supply chains, extended lockdowns, and cancelling of travel visits of sales personnel of companies have also negativity affected the growth of the arrhythmia monitoring devices market.

Strategic insights for the Europe Arrythmia Monitoring Devices provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
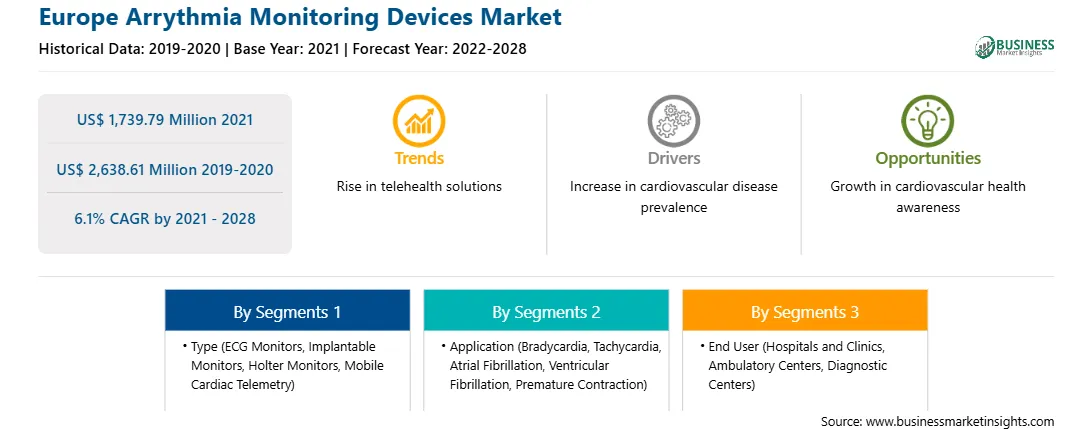
| Market size in 2021 | US$ 1,739.79 Million |
| Market Size by 2028 | US$ 2,638.61 Million |
| Global CAGR (2021 - 2028) | 6.1% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe Arrythmia Monitoring Devices refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The arrythmia monitoring devices market in Europe is expected to grow from US$ 1,739.79 million in 2021 to US$ 2,638.61 million by 2028; it is estimated to grow at a CAGR of 6.1% from 2021 to 2028. Market consolidations, Changes or shift in technology-related trends encourage players to develop innovative devices. Recent developments in HIPPA-compliant technology have enabled secured data transmission throughout the monitoring period without delays. Emerging technologies are providing effective alternatives for traditional Holter monitors that create alerts for patients with a high risk of cardiac arrhythmia. Hence, continuous remote monitoring capabilities of these devices facilitating long-term, uninterrupted cardiac rhythm management are adding to their popularity. The mobile cardiac telemetry (MCT) system is an innovative product that assist in monitoring heart conditions of cardiac patients. Patients carry the tiny sensor and monitor throughout the day, and whenever, a cardiac event occurs, MCT sends the data to a central system for analysis and reaction. The system generates a report and shares it with the patient's physician, along with graphs and trends, for further actions. The abilities of MCT systems to analyze every heartbeat with little interference to the patient's normal days and to initiate an immediate emergency response as needed make them one of the most attractive choices cardiac monitoring. This is bolstering the growth of the arrythmia monitoring devices market.
In terms of type, the holter monitors segment accounted for the largest share of the Europe arrythmia monitoring devices market in 2020. In terms of application, the atrial fibrillation segment held a larger market share of the arrythmia monitoring devices market in 2020. Further, the hospitals and clinics segment held a larger share of the market based on end user in 2020.
A few major primary and secondary sources referred to for preparing this report on arrythmia monitoring devices market in Europe are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Abbott
The Europe Arrythmia Monitoring Devices Market is valued at US$ 1,739.79 Million in 2021, it is projected to reach US$ 2,638.61 Million by 2028.
As per our report Europe Arrythmia Monitoring Devices Market, the market size is valued at US$ 1,739.79 Million in 2021, projecting it to reach US$ 2,638.61 Million by 2028. This translates to a CAGR of approximately 6.1% during the forecast period.
The Europe Arrythmia Monitoring Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe Arrythmia Monitoring Devices Market report:
The Europe Arrythmia Monitoring Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe Arrythmia Monitoring Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe Arrythmia Monitoring Devices Market value chain can benefit from the information contained in a comprehensive market report.