3D printers are used to manufacture a variety of medical devices, including those with complex geometry or features that match a patient’s unique anatomy. A few devices are printed from a standard design, and then multiple identical copies of the same device are made. Other devices, called patient-matched or patient-specific devices, are created from the patient-specific imaging data. The choice of technology used for 3D printing depends on many factors, including the intended use of printed products and the simplicity of the printer, among others. Powder bed fusion is the most common technology used for the 3D printing of medical devices. This technique is compatible with various materials used in medical devices, such as titanium and nylon. With 3D printing, creating patient-specific, tactile reference models from CT and MRI scans is both affordable and simple. These models provide an additional perspective that helps physicians better prepare for surgeries, resulting in drastically reduced time and cost of an actual procedure performed within an operating room. This may benefit patients through greater satisfaction, lowered anxiety, and shortened recovery time. In addition to this, the launch of new biocompatible medical 3D printing materials helps in the development of new surgical tools and techniques with the ultimate goal of improving the clinical experience during surgery. The tools that can be printed using 3D printing techniques include sterilizable fixation trays, contouring templates, and implant sizing models, which can be used to size implants in the operating room before the first cut, allowing surgeons to save time and improve accuracy during complex procedures.
Due to the coronavirus outbreak, the European Commission decided to push back the transition from MDD to MDR. This permits the medical device sector and Notified Bodies (NBs) to concentrate on the current issue and devote all of their resources to combatting the coronavirus. However, there has been no indication on whether the In Vitro Diagnostic Regulation (IVDR) 2017/246, which is set to take effect in May 2021 would be postponed. This adds to the strain on IVD makers, who must stay on track with the improvements required to move to IVDR. They must also deal with the increased demand for key diagnostic tests as well as the difficulty of kit manufacture and distribution during the pandemic. The European Commission has temporarily made the export of certain medical gadgets, such as personal protective equipment (PPE) and other similar protective gear, subject to authorization. Medical device companies that are contractually obligated to supply devices to nations outside of the EU may find this problematic. Norway, Lichtenstein, and Switzerland are exempt from this authorization.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the 3D printing medical devices market. The Europe 3D printing medical devices market is expected to grow at a good CAGR during the forecast period.
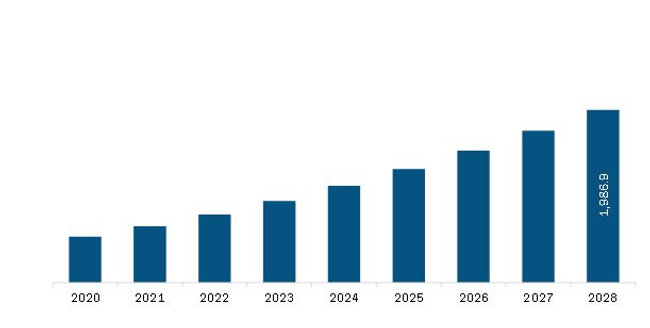
Europe 3D Printing Medical Devices Market Revenue and Forecast to 2028 (US$ Million)
Strategic insights for the Europe 3D Printing Medical Devices provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
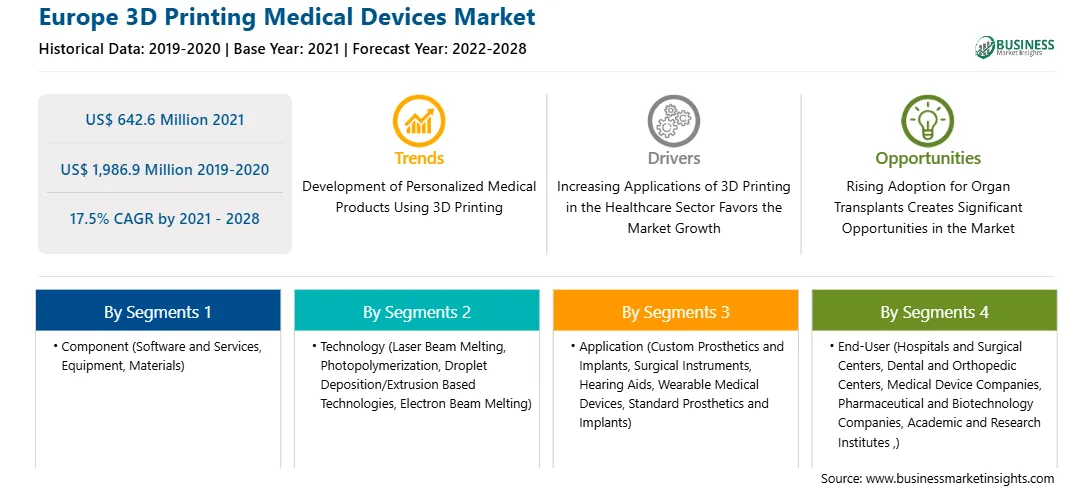
| Market size in 2021 | US$ 642.6 Million |
| Market Size by 2028 | US$ 1,986.9 Million |
| Global CAGR (2021 - 2028) | 17.5% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Component
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
The geographic scope of the Europe 3D Printing Medical Devices refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The Europe 3D Printing Medical Devices Market is valued at US$ 642.6 Million in 2021, it is projected to reach US$ 1,986.9 Million by 2028.
As per our report Europe 3D Printing Medical Devices Market, the market size is valued at US$ 642.6 Million in 2021, projecting it to reach US$ 1,986.9 Million by 2028. This translates to a CAGR of approximately 17.5% during the forecast period.
The Europe 3D Printing Medical Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Europe 3D Printing Medical Devices Market report:
The Europe 3D Printing Medical Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Europe 3D Printing Medical Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Europe 3D Printing Medical Devices Market value chain can benefit from the information contained in a comprehensive market report.