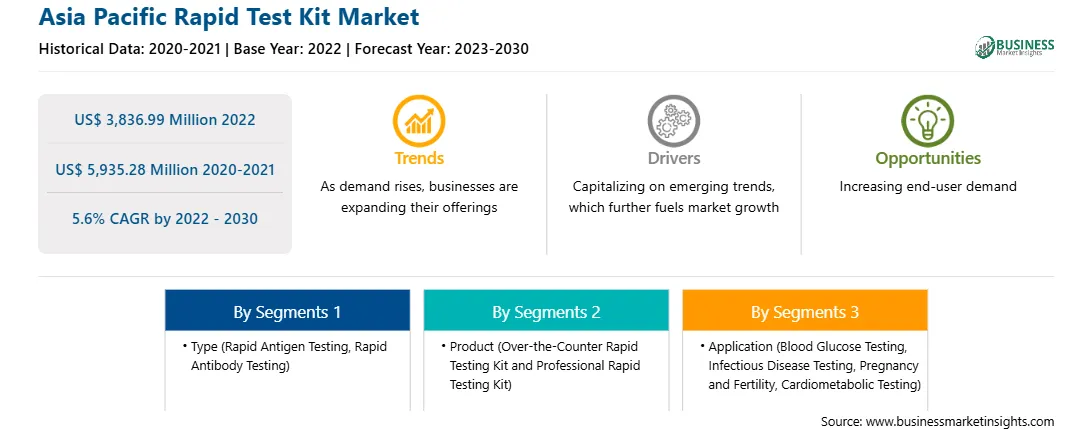
The Asia Pacific rapid test kit market was valued at US$ 3,836.99 million in 2022 and is expected to reach US$ 5,935.28 million by 2030; it is estimated to record a CAGR of 5.6% from 2022 to 2030. Increasing Preference for PCR Techniques Drives Asia Pacific Rapid Test Kit Market
In recent years, molecular methods such as PCR have evolved as more effective diagnostic tools, as they aid in the precise diagnosis of a wide range of infectious diseases. Most of the popular molecular tests use reverse transcription polymerase chain reaction (RT-PCR). The technique provides reproducible results that are comparable between different laboratories and hence are accepted worldwide. The manufacturers are involved in new PCR developments.
For instance, GeneSoC is a recently developed system in which the PCR reaction solutions are reciprocally driven over numerous preheated zones and encapsulated on a chip within an extremely thin flow channel. GeneSoC is available for multiplex real-time PCR and is capable of concurrently detecting 3 fluorescence channels. It is regarded as an excellent alternative diagnostic technology for the detection of influenza A and B viruses, as it completes the real-time RT-PCR reaction in ~16 minutes. PCR techniques are also being widely accepted and utilized in emerging countries. According to a report by the World Health Organization on the Establishment of PCR Laboratory in Developing Countries 2016, WHO provides necessary technical assistance to establish PCR laboratories in all 11 WHO Southeast Asia countries. These facilities have adopted different PCR technologies such as RT-PCR, multiplex PCR, real-time PCR, in-situ PCR, and dPCR, depending on their needs. The application of PCR technologies is also increasing in clinical microbiology, virology, parasitology, biotechnology, and allied fields due to the simple, rapid, and highly specific screening enabled by this tool. Thus, the growing preference for PCR as a diagnostic and clinical tool is expected to provide lucrative opportunities to the rapid test kits market during the forecast period.Asia Pacific Rapid Test Kit Market Overview
The World Health Organization considers HIV self-testing (HIVST) to be a legitimate alternative to standard HIV testing performed in diagnostic laboratories. Over the last five years, the healthcare sector and authorities in China have strived to gradually increase the extent of HIVST use. The coverage of HIV testing can be further increased with the help of HIVST to identify the condition among individuals living with HIV but are ignorant of their infection. The Chinese government emphasizes on the rapidly rising HIVST rate among homosexual men. The country has been exploring strategies to promote HIV self-testing through selling HIVST kits in pharmacies and online, according to the State Council of China, which released the "Thirteenth Five-Year Plan (2017-2022)" for HIV prevention and control in 2017.
Asia Pacific Rapid Test Kit Market Revenue and Forecast to 2030 (US$ Million)
Strategic insights for the Asia Pacific Rapid Test Kit provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Asia Pacific Rapid Test Kit refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.Asia Pacific Rapid Test Kit Strategic Insights
Asia Pacific Rapid Test Kit Report Scope
Report Attribute
Details
Market size in 2022
US$ 3,836.99 Million
Market Size by 2030
US$ 5,935.28 Million
Global CAGR (2022 - 2030)
5.6%
Historical Data
2020-2021
Forecast period
2023-2030
Segments Covered
By Type
By Product
By Application
By End User
Regions and Countries Covered
Asia-Pacific
Market leaders and key company profiles
Asia Pacific Rapid Test Kit Regional Insights
Asia Pacific Rapid Test Kit Market Segmentation
The Asia Pacific rapid test kit market is segmented based on type, product, technology, application, end user, and country. Based on type, the Asia Pacific rapid test kit market is segmented into rapid antigen testing, rapid antibody testing, and others. The rapid antigen testing segment held the largest market share in 2022.
In terms of product, the Asia Pacific rapid test kit market is bifurcated into over-the-counter rapid testing kit and professional rapid testing kit. The over-the-counter rapid testing kit segment held a larger market share in 2022.
By technology, the Asia Pacific rapid test kit market is segmented into lateral flow assay, solid phase, agglutination, immunospot assay, and cellular component-based. The lateral flow assay segment held the largest market share in 2022.
Based on application, the Asia Pacific rapid test kit market is categorized into blood glucose testing, infectious disease testing, pregnancy and fertility, cardiometabolic testing, and others. The blood glucose testing segment held the largest market share in 2022.
In terms of end user, the Asia Pacific rapid test kit market is segmented into hospital and clinics, home care, diagnostics centers, and others. The hospital and clinics segment held the largest market share in 2022.
Based on country, the Asia Pacific rapid test kit market is segmented into China, India, Japan, South Korea, Australia, and the Rest of Asia Pacific. China dominated the Asia Pacific rapid test kit market share in 2022.
F. Hoffmann-La Roche Ltd, Becton Dickinson and Co, ARKRAY Inc, Fujirebio Europe NV, bioMerieux SA, Cepheid, Meril Life Sciences Pvt Ltd, QIAGEN NV, OraSure Technologies Inc, Guangzhou Wondfo Biotech Co Ltd, Denka Co Ltd, Abbott Laboratories, SD Biosensor Inc, Premier Medical Corp Pvt Ltd, Bio-Rad Laboratories Inc, Hologic Inc, DiaSorin SpA, and Beckman Coulter Inc are some of the leading players operating in the Asia Pacific rapid test kit market.
1. F. Hoffmann-La Roche Ltd
2. Becton Dickinson and Co
3. ARKRAY Inc
4. Fujirebio Europe NV
5. bioMerieux SA
6. Cepheid
7. Meril Life Sciences Pvt Ltd
8. QIAGEN NV
9. OraSure Technologies Inc
10. Guangzhou Wondfo Biotech Co Ltd
11. Denka Co Ltd
12. Abbott Laboratories
13. Bio-Rad Laboratories Inc
14. Hologic Inc
15. SD Biosensor Inc
16. Premier Medical Corp Pvt Ltd
17. DiaSorin SpA
18. Beckman Coulter Inc
The Asia Pacific Rapid Test Kit Market is valued at US$ 3,836.99 Million in 2022, it is projected to reach US$ 5,935.28 Million by 2030.
As per our report Asia Pacific Rapid Test Kit Market, the market size is valued at US$ 3,836.99 Million in 2022, projecting it to reach US$ 5,935.28 Million by 2030. This translates to a CAGR of approximately 5.6% during the forecast period.
The Asia Pacific Rapid Test Kit Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Rapid Test Kit Market report:
The Asia Pacific Rapid Test Kit Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Rapid Test Kit Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Rapid Test Kit Market value chain can benefit from the information contained in a comprehensive market report.