APAC is the fastest-growing market for POC diagnostics, and China, India, Japan, South Korea, Australia, and rest of APAC are the major contributors to the market in this region. China is the largest market of APAC region. The government of China has adopted various comprehensive strategies, including activities such as blood donation screening, promotion of safe injection practices, immunization, and surveillance, to prevent HBV transmission. The China Centers for Disease Control and Prevention also believes that the application of proper infection control practice can lead to success in the prevention of HAIs. However, at the level of average Chinese hospitals, there is no vivid understanding regarding the surveillance of infection and programs to detect, manage, and prevent these HAIs. Many companies have emerged in the country with innovative products and other activities to propel market growth. For instance, in December 2018, Abcam plc. and QIAGEN (Suzhou) Translational Medicine Co., Ltd. signed a Memorandum of Understanding (MoU) agreement. The agreement aims to develop a pipeline of Companion Diagnostics (CDx) as well as in vitro diagnostic reagents and kits to meet the needs of the Chinese market. Moreover, in January 2019, Ascensia Diabetes Care, a Swiss-based diagnostics company started its operations in China in collaboration with China-based Zhejiang POCTech. The new device is continuous blood glucose monitoring and incorporates modules for digital diabetes management. Furthermore, in October 2018, Cancer Genetics, Inc., signed a distribution agreement with Genecast Biotechnology to market, distribute, and sell the Tissue of Origin (TOO) Test in China. Due to the rising prevalence of diseases and increasing government initiatives in China, the POC diagnostics market is anticipated to grow with a rapid pace during the forecast period.
In case of COVID-19, APAC is highly affected specially India. Many measures have been implemented to contain the spread of COVID-19, which have resulted in significant operational disruption for many companies including in the healthcare industry. Staff quarantine, supply-chain failures, and reductions in demand have generated serious complications for companies. Rising prevalence and incidence of COVID-19 support the growth of the market in near future forecast period. Increasing launch of PCR tests for detection COVID-19 support the growth of the market. In April 2021, Fujita Health University in Toyoake has started using a fully automated coronavirus testing system from Kawasaki Heavy Industries Ltd. The machine conducts polymerase chain reaction (PCR) test to reducing the risk of infection for technicians. Therefore, the outbreak of the COVID-19 pandemic is estimated to affect high impact on molecular biology enzymes, kits & reagents industry. Moreover, in June 2020, J Mitra & Co Pvt Ltd. an India-based company introduced its state-of-the-art COVID-19 detection kit in Indian market. Further, in May 2021, Mylab Discovery solutions, India-based company introduced home testing kits for Covid-19. The kit incorporates reverse transcription polymerase chain reaction (RT-PCR) technology. Besides, in April 2021, Celltrion, a South Korea based company announced approval and launch of its DiaTrust rapid antigen test for diagnosis of COVID-19. The kit has capacity to deliver results within 15 minutes. Also, in April 2020, India-based company Bione announced launch of India’s first home screening kit for diagnosis of COVID-19. Such product launches and approval to boost market growth during the pandemic in APAC.

Strategic insights for the Asia Pacific Point of Care Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
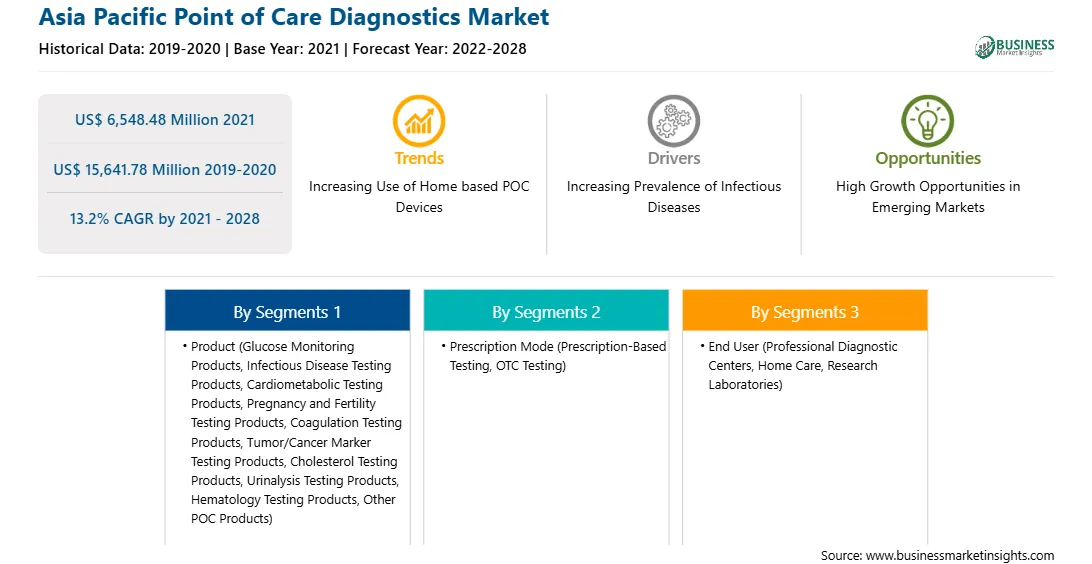
| Market size in 2021 | US$ 6,548.48 Million |
| Market Size by 2028 | US$ 15,641.78 Million |
| Global CAGR (2021 - 2028) | 13.2% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
The geographic scope of the Asia Pacific Point of Care Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The APAC point of care diagnostics market is expected to grow from US$ 6,548.48 million in 2021 to US$ 15,641.78 million by 2028; it is estimated to grow at a CAGR of 13.2% from 2021 to 2028. Point-of-care testing (POCT) plays a critical role in diagnosis, treatment, and prevention of infectious diseases. POC testing can be used to detect several major pathogens, including malarial parasites, human immunodeficiency virus (HIV), human papillomavirus (HPV), dengue, Ebola, and Zika viruses; and Mycobacterium tuberculosis (TB bacteria). The increasing incidence of infectious diseases such as HIV is the major public health issue. Moreover, healthcare-associated infections (HAIs) such as central line-associated bloodstream infections and catheter-associated urinary tract infections might affect the patients in hospitals and other healthcare facilities. Such increase in prevalence of infectious diseases is expected to create a demand for point of care test kits across the APAC region.
In terms of product, the glucose monitoring products segment accounted for the largest share of the APAC point of care diagnostics market in 2020. In terms of prescription mode, the prescription-based testing segment held a larger market share of the APAC point of care diagnostics market in 2020. Further, the professional diagnostic centers segment held a larger share of the APAC point of care diagnostics market based on end user in 2020.
A few major primary and secondary sources referred to for preparing this report on the APAC point of care diagnostics market are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Abbott; BD; bioMerieux SA; BIO-RAD LABORATORIES INC.; Danaher; F. HOFFMANN-LA ROCHE LTD.; Johnson and Johnson Services, Inc.; Nova Biomedical; Polymer Technology Systems, Inc. (PTS); and Siemens AG.
The Asia Pacific Point of Care Diagnostics Market is valued at US$ 6,548.48 Million in 2021, it is projected to reach US$ 15,641.78 Million by 2028.
As per our report Asia Pacific Point of Care Diagnostics Market, the market size is valued at US$ 6,548.48 Million in 2021, projecting it to reach US$ 15,641.78 Million by 2028. This translates to a CAGR of approximately 13.2% during the forecast period.
The Asia Pacific Point of Care Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Point of Care Diagnostics Market report:
The Asia Pacific Point of Care Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Point of Care Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Point of Care Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.