Asia Pacific Pharmacovigilance and Drug Safety Software Market
No. of Pages: 129 | Report Code: TIPRE00022770 | Category: Technology, Media and Telecommunications
No. of Pages: 129 | Report Code: TIPRE00022770 | Category: Technology, Media and Telecommunications
Pharmacovigilance (PV) plays an essential role in the healthcare system through assessment, monitoring, and finding of drug interactions and their effects in human. Pharmacovigilance helps companies to monitor any adverse drug reaction events during the trial phase and also during the post marketing period.
Thus, the globalization of pharmacovigilance are expected to create a significant demand for pharmacovigilance and drug safety software in the coming years, which is further anticipated to drive the pharmacovigilance and drug safety software market.
Countries in Asia-Pacific are expecting to witness huge challenge due to growing COVID-19. Considering the economic operations and geographic condition, the outbreak of disease has affected on medical tourism, manufacturer of medical equipment, laser systems, accessories and other problems posed by shortage of healthcare infrastructure in Asia-Pacific low-income countries. After the first case in December in Wuhan, China, the coronavirus has spread to at least 180 countries and regions. To prevent the spread of disease, restrictive measures have been taken in countries such as India, South Korea, Singapore, Malaysia, and by the Philippines. According to WHO, due to the rapidly changing risk of COVID-19 affected countries and constantly controlling outbreak trends, any additional health measures are likely to significantly interfere with international travel and trade. There is vast variation in the reporting standards across the Asia Pacific. While some countries have adopted electronic reporting, many organizations still use conventional methods of courier or hand delivery for paper reporting or electronic reporting via compact disc. Currently, electronic reporting and email submissions are compatible with COVID-19. However, all methods require efficient communication between pharmacovigilance teams, local legal representative, courier support and teams dispersed across various geographical locations, for example portal entry in the local language.
Strategic insights for the Asia Pacific Pharmacovigilance and Drug Safety Software provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
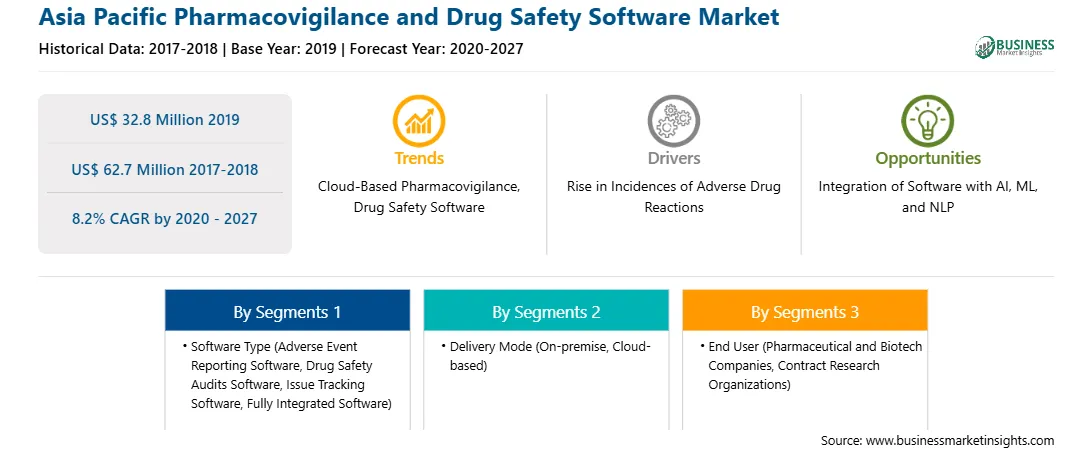
| Market size in 2019 | US$ 32.8 Million |
| Market Size by 2027 | US$ 62.7 Million |
| Global CAGR (2020 - 2027) | 8.2% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Software Type
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
The geographic scope of the Asia Pacific Pharmacovigilance and Drug Safety Software refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
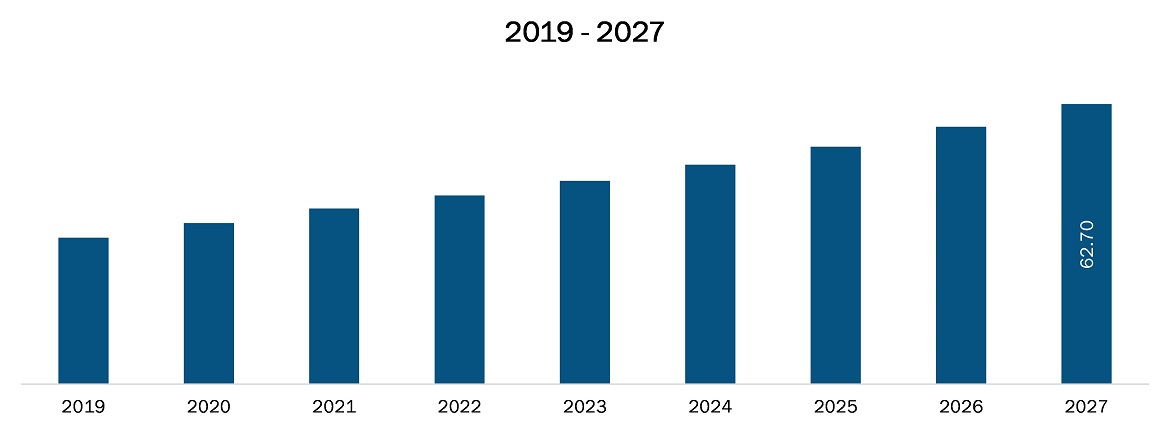
The pharmacovigilance and drug safety software market in Asia Pacific is expected to grow from US$ 32.8 million in 2019 to US$ 62.7 million by 2027; it is estimated to grow at a CAGR of 8.2% from 2020 to 2027. Adverse drug reactions (ADRs) is an important public health problem, signifying a significant cause of illness and death. Because all drugs have the potential for adverse drug reactions, a risk-benefit analysis is necessary whenever a drug is prescribed. ADR reported by a patient or healthcare professional adds to safety monitoring and thus to the safe and effective use of medicines. The increasing amount of data generated through adverse drug reaction report need to be handled and stored carefully. All these data come in different forms, language, location, etc. To arrange these uniformly the automation systems/software are helpful. Thus, the rising incidences of adverse drug reactions (ADRs) are likely to drive the market’s growth.
In terms of software type, the adverse event reporting software segment accounted for the largest share of the Asia Pacific pharmacovigilance and drug safety software market in 2019. In terms of delivery mode, the on-premise segment held a larger market share of the pharmacovigilance and drug safety software market in 2019. In terms of end user, the contract research organizations segment held a larger market share of the pharmacovigilance and drug safety software market in 2019.
A few major primary and secondary sources referred to for preparing this report on the Pharmacovigilance and drug safety software market in Asia Pacific are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Veeva Systems
By Software Type
By Delivery Mode
By End User
By Country
The Asia Pacific Pharmacovigilance and Drug Safety Software Market is valued at US$ 32.8 Million in 2019, it is projected to reach US$ 62.7 Million by 2027.
As per our report Asia Pacific Pharmacovigilance and Drug Safety Software Market, the market size is valued at US$ 32.8 Million in 2019, projecting it to reach US$ 62.7 Million by 2027. This translates to a CAGR of approximately 8.2% during the forecast period.
The Asia Pacific Pharmacovigilance and Drug Safety Software Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Pharmacovigilance and Drug Safety Software Market report:
The Asia Pacific Pharmacovigilance and Drug Safety Software Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Pharmacovigilance and Drug Safety Software Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Pharmacovigilance and Drug Safety Software Market value chain can benefit from the information contained in a comprehensive market report.