Molecular diagnostic techniques and platforms are being used in all areas of anatomic and clinical pathologies. DNA or RNA sequences that are associated with disease, including single nucleotide polymorphism, deletions, rearrangements, and insertions, can be detected in molecular diagnostic tests. Molecular diagnosis has undergone further improvements after the emergence of COVID-19. Conventionally, CT scans, hematological tests, and RT-PCR were in use for testing. As COVID-19 cases grew rapidly in 2020, the need for rapid, precise testing platforms surged to overcome the disadvantages of conventional testing. Computed tomography (CT), a cost-intensive procedure that may not be available in all hospitals, fails to detect viral infections and other diseases in asymptomatic patients. RT-PCR, which was widely used, was also time-consuming and expensive, and was unable to detect a low viral load during the early stages of infection. Consequently, researchers developed novel approaches to detect SARS-CoV-2, which were faster and more cost effective. Reverse transcription loop-mediated isothermal amplification (RT-LAMP), microarray-based detection, aptamer-based diagnosis, SHERLOCK, SHERLOCKv2, FET Biosensors, cell-based potentiometric diagnosis, and molecular imprinting technology are a few of the examples of novel molecular diagnostics techniques developed for COVID-19 diagnosis. In the last few years, FDA reports have indicated that advancements in molecular testing, antigen-dependent testing, and serological testing have been approved. Collaborative efforts by scientific communities in different countries to manage the COVID-19 pandemic and reduce the extent of mortality have benefited the overall molecular tools and diagnosis landscape, which is likely to create significant opportunities for the molecular diagnostics market in the future.
Asia Pacific includes Malaysia, Thailand, India, Singapore, the Philippines, Vietnam, Indonesia, China, Japan, Australia, South Korea, and the Rest of Asia Pacific. Rising geriatric population, increasing cancer cases, growing technological advancements, and increasing number of startups, biotechnology, and biopharmaceutical companies are driving the molecular diagnostics market in this region. Moreover, the rise in research activities in the region and the presence of associations or organizations enhancing the quality of care in cancer contribute to the molecular diagnostics market growth.
Asia Pacific Molecular Diagnostics Market Revenue and Forecast to 2030 (US$ Million)
The Asia Pacific molecular diagnostics market is segmented into disease area, technology, product and services, end user, and country.
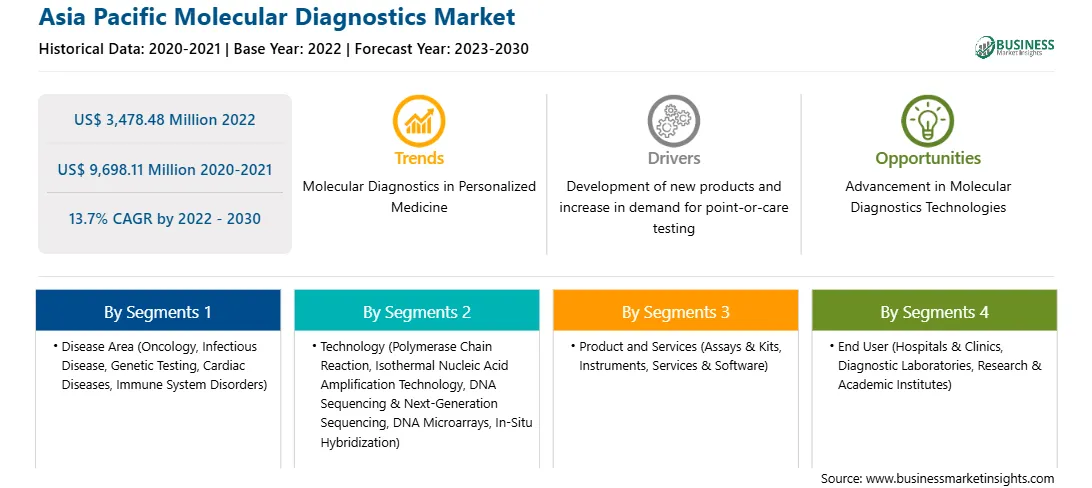
Based on disease area, the Asia Pacific molecular diagnostics market is segmented into infectious disease, oncology, genetic testing, cardiac diseases, immune system disorders, and others. The infectious disease segment registered the largest Asia Pacific molecular diagnostics market share in 2022.
Based on technology, the Asia Pacific molecular diagnostics market is segmented into polymerase chain reaction (PCR), in situ hybridization, isothermal nucleic acid amplification technology (INAAT), DNA sequencing and next-generation sequencing (NGS), DNA microarrays, and others. The polymerase chain reaction (PCR) segment registered the largest Asia Pacific molecular diagnostics market share in 2022. The polymerase chain reaction (PCR) is further sub segmented into RT-PCR, qPCR, multiplex PCR, and others.
Based on product and services, the Asia Pacific molecular diagnostics market is segmented into assays and kits, instruments, and services and software. The assays and kits segment registered the largest Asia Pacific molecular diagnostics market share in 2022.
Based on end users, the Asia Pacific molecular diagnostics market is segmented into diagnostic laboratories, hospitals and clinics, research and academic institutes, and others. The diagnostic laboratories segment held the largest Asia Pacific molecular diagnostics market share in 2022.
Based on country, the Asia Pacific molecular diagnostics market has been categorized into India, Singapore, Malaysia, Philippines, Thailand, Indonesia, Vietnam, China, Japan, Australia, South Korea, and the Rest of Asia Pacific. China dominated the Asia Pacific molecular diagnostics market in 2022.
Abbott Laboratories, Agilent Technologies Inc, Thermo Fisher Scientific Inc, F. Hoffmann-La Roche Ltd, Qiagen NV, bioMerieux SA, Illumina Inc, Danaher, Siemens Healthineers AG, Novartis AG, and TBG Diagnostics Limited are some of the leading companies operating in the Asia Pacific molecular diagnostics market.
Strategic insights for the Asia Pacific Molecular Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 3,478.48 Million |
| Market Size by 2030 | US$ 9,698.11 Million |
| Global CAGR (2022 - 2030) | 13.7% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Disease Area
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
The geographic scope of the Asia Pacific Molecular Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The Asia Pacific Molecular Diagnostics Market is valued at US$ 3,478.48 Million in 2022, it is projected to reach US$ 9,698.11 Million by 2030.
As per our report Asia Pacific Molecular Diagnostics Market, the market size is valued at US$ 3,478.48 Million in 2022, projecting it to reach US$ 9,698.11 Million by 2030. This translates to a CAGR of approximately 13.7% during the forecast period.
The Asia Pacific Molecular Diagnostics Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Molecular Diagnostics Market report:
The Asia Pacific Molecular Diagnostics Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Molecular Diagnostics Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Molecular Diagnostics Market value chain can benefit from the information contained in a comprehensive market report.