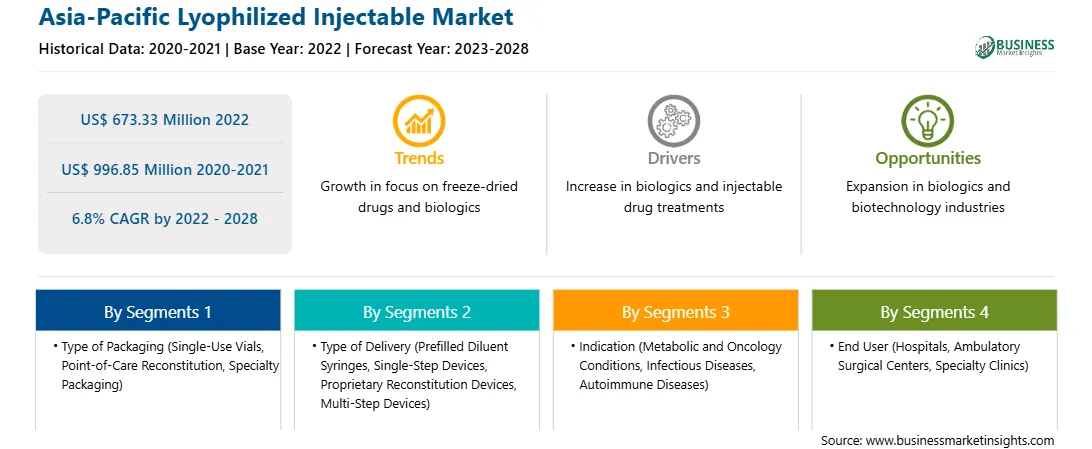
The APAC lyophilized injectable market is expected to grow from US$ 673.33 million in 2022 to US$ 996.85 million by 2028. It is estimated to grow at a CAGR of 6.8% from 2022 to 2028.
Advancement in Lyophilized Packaging
The complexity of drug reconstitution constrained the market potential for lyophilized drugs and vaccines. Multiple pieces of device and long procedures are required to reconstitute a lyophilized drug from a vial; thus, most of these products are restricted within healthcare facilities. However, the healthcare transformation toward patient self-injection and the acceleration after biologics flared a new wave of creation for drug reconstitution technology. Dual-chamber syringes represent an enabling technology for the self-injection of lyophilized drugs and new therapies directing the mixing of two liquid molecules at the time of drug delivery. Prefilled dual chamber devices (DCDs) are combination devices containing freeze-dried drugs and diluent in two separate chambers of the device. DCDs have shown many advantages, such as easy handling, accurate dosing, seal integrity, and high stability, compared to the traditional drug-delivery systems; therefore, they are promising alternatives to these traditional drug-delivery systems. DCDs are being increasingly preferred by physicians for patients needing instant relief and safe delivery, significantly improving drug delivery by quickly applying pressure to the end plunger after mixing and dissolving freeze-dried powder. DCDs can improve long-term pharmaceutical stability by storing them in the lyophilized form before administration and therefore are becoming more popular, especially for those unstable pharmaceutical products. Therefore, advancement in lyophilized packaging is expected to drive the market across the region.
Market Overview
The APAC lyophilized injectable market is segmented into China, India, Japan, South Korea, Australia, and the Rest of APAC. China dominated the market in 2022. The market's growth is primarily attributed to the established pharmaceutical market, increased R&D expenditures by pharmaceutical and biopharmaceutical companies, and favorable regulatory policies. The excessive population in China and the rise of various communicable and noncommunicable diseases are majorly driving the pharmaceutical companies in China, making it a prime market in Asia Pacific. For instance, the COVID-19 outbreak in China led to the launch of several drugs to counter the virus. It increased contract manufacturing of drugs, such as lyophilized remdesivir powder, fueling the demand for lyophilized injectables in the country. Due to the pandemic, various market players ramped up the production of certain lyophilized drugs as it ensures safe and prolonged packaging of the drugs. Market players in China have constantly been evolving with organic and inorganic strategies. For instance, WuXi STA—a subsidiary of WuXi AppTec—announced its first parenteral formulation production line at the Wuxi city site. This wholly automatic sterile manufacturing line runs in a complete isolation system with an annual capacity of 2 million units. The automatic parenteral filling line features numerous filling modules and a built-in lyophilizer to fill syringes, vials, and cartridges of a full range of sizes and produce lyophilized powders. Thus, growing pharmaceutical & biopharmaceutical market and increasing initiatives by market players are expected to drive the market during the forecast period.
Strategic insights for the Asia-Pacific Lyophilized Injectable provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 673.33 Million |
| Market Size by 2028 | US$ 996.85 Million |
| Global CAGR (2022 - 2028) | 6.8% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Type of Packaging
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
The geographic scope of the Asia-Pacific Lyophilized Injectable refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
APAC Lyophilized Injectable Market Segmentation
The APAC lyophilized injectable market is segmented based on type of packaging, type of delivery, indication, end user, and country.
Aristopharma Ltd.; Baxter; CordenPharma International; Credence MedSystems, Inc.; Curia Global, Inc.; Jubilant HollisterStier (Jubilant Pharma Limited); Nipro; Recipharm AB; S.G. Biopharm Pvt. Ltd.; and Vetter Pharma are among the leading companies operating in the APAC lyophilized injectable market.
The Asia-Pacific Lyophilized Injectable Market is valued at US$ 673.33 Million in 2022, it is projected to reach US$ 996.85 Million by 2028.
As per our report Asia-Pacific Lyophilized Injectable Market, the market size is valued at US$ 673.33 Million in 2022, projecting it to reach US$ 996.85 Million by 2028. This translates to a CAGR of approximately 6.8% during the forecast period.
The Asia-Pacific Lyophilized Injectable Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia-Pacific Lyophilized Injectable Market report:
The Asia-Pacific Lyophilized Injectable Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia-Pacific Lyophilized Injectable Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia-Pacific Lyophilized Injectable Market value chain can benefit from the information contained in a comprehensive market report.