Biopharmaceutical companies in the lyophilization services market are launching advanced lyophilization technologies to expand their business and maintain a competitive environment in the market. In August 2022, Leadgene Biomedical, Inc., an ISO-certificated contract development and manufacturing organization (CDMO) providing integrated services for in-vitro diagnostics (IVD) products from the development to manufacturing process, launched LEADSPHERE Lyophilization Technology. The technology aids in dispensing, storing, and transporting reagents for use in biomolecule analysis tools. It also allows assay reagents to be stable in the nonliquid form at room temperature and enables the assembling of the complete kit in a large-scale production manner through a 'pick and place' automation system. A successful example that uses LEADSPHERE Lyophilization Technology is the LEADSPHERE Proteinase K, an endopeptidase used for nucleic acids sample preparation by inactivating ribonuclease and digesting proteins. LEADSPHERE Proteinase K has been used in applications to detect viruses, such as SARS-CoV-2.
In May 2022, PCI Pharma Services (PCI), a leading global CDMO, announced a major expansion of capacity and capabilities in sterile lyophilization technology and aseptic liquid fill-finish—an important manufacturing process commonly used with injectable and biologic therapies—with an investment of US$ 100 million in the construction and enhancement of world-class facilities at its Bedford, New Hampshire campus. The facility will contain state-of-the-art technology, including an aseptic fill-finish line with a completely isolated containment system. Also, it will hold twin lyophilizers with autoloading and unloading systems, with the capacity to complete 400 vials/minute on a sterile fill-finish line.
The launch of such advanced lyophilization technologies is likely to offer opportunities for players operating in the Asia Pacific lyophilization services for biopharmaceuticals market to attain a significant position in the coming years.
Asia Pacific is the fastest-growing market for lyophilization services for biopharmaceuticals globally. The market in Asia Pacific is segmented into China, India, Japan, Australia, South Korea, and the Rest of Asia Pacific. China, Japan, and India widely contribute to market growth. The increasing production of biopharmaceutical products is likely to demand lyophilization services in these countries. Also, the rising investments in domestic companies are expected to influence the market growth during the forecast period.
On the other hand, countries such as South Korea and Australia serve vital growth opportunities due to rising clinical trials. Growing investment in research and development is likely to contribute to market growth.
Strategic insights for the Asia Pacific Lyophilization Services for Biopharmaceuticals provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Asia Pacific Lyophilization Services for Biopharmaceuticals refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.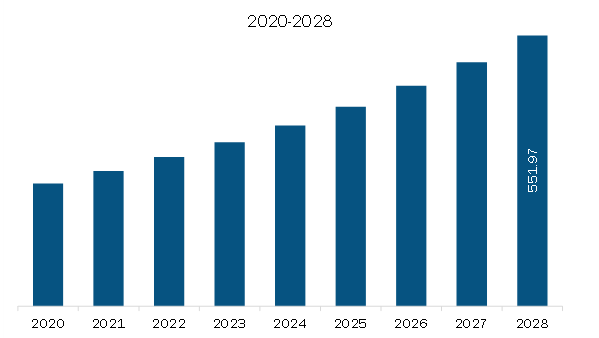
Asia Pacific Lyophilization Services for Biopharmaceuticals Strategic Insights
Asia Pacific Lyophilization Services for Biopharmaceuticals Report Scope
Report Attribute
Details
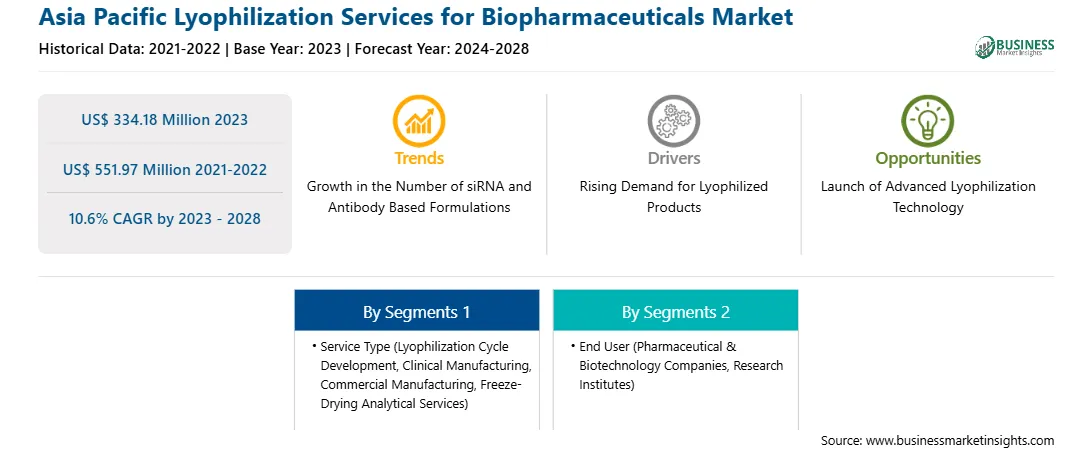
Market size in 2023
US$ 334.18 Million
Market Size by 2028
US$ 551.97 Million
Global CAGR (2023 - 2028)
10.6%
Historical Data
2021-2022
Forecast period
2024-2028
Segments Covered
By Service Type
By End User
Regions and Countries Covered
Asia-Pacific
Market leaders and key company profiles
Asia Pacific Lyophilization Services for Biopharmaceuticals Regional Insights
Asia Pacific Lyophilization Services for Biopharmaceuticals Market Segmentation
The Asia Pacific lyophilization services for biopharmaceuticals market is segmented into service type, end user, and country.
Based on service type, the Asia Pacific lyophilization services for biopharmaceuticals market is segmented into lyophilization cycle development, clinical manufacturing, commercial manufacturing, and freeze-drying analytical services. The commercial manufacturing segment held the largest share of the Asia Pacific lyophilization services for biopharmaceuticals market in 2023.
By end user, the Asia Pacific lyophilization services for biopharmaceuticals market is segmented into pharmaceutical & biotechnology companies, research institutes, and others. The pharmaceutical & biotechnology companies segment held the largest share of the Asia Pacific lyophilization services for biopharmaceuticals market in 2023.
Based on country, the Asia Pacific lyophilization services for biopharmaceuticals market is segmented into China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific. China dominated the Asia Pacific lyophilization services for biopharmaceuticals market in 2023.
Curia Global Inc, Emergent BioSolutions Inc, Jubilant HollisterStier LLC., and PCI Pharma Services are the leading companies operating in the Asia Pacific lyophilization services for biopharmaceuticals market.
The Asia Pacific Lyophilization Services for Biopharmaceuticals Market is valued at US$ 334.18 Million in 2023, it is projected to reach US$ 551.97 Million by 2028.
As per our report Asia Pacific Lyophilization Services for Biopharmaceuticals Market, the market size is valued at US$ 334.18 Million in 2023, projecting it to reach US$ 551.97 Million by 2028. This translates to a CAGR of approximately 10.6% during the forecast period.
The Asia Pacific Lyophilization Services for Biopharmaceuticals Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Lyophilization Services for Biopharmaceuticals Market report:
The Asia Pacific Lyophilization Services for Biopharmaceuticals Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Lyophilization Services for Biopharmaceuticals Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Lyophilization Services for Biopharmaceuticals Market value chain can benefit from the information contained in a comprehensive market report.