Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing Market
No. of Pages: 129 | Report Code: BMIRE00027537 | Category: Life Sciences
No. of Pages: 129 | Report Code: BMIRE00027537 | Category: Life Sciences
Spreading Awareness Among Individuals Regarding Diagnosis of H. pylori
Diagnosis of H. pylori infection is performed by invasive (endoscopy and endoscopic biopsy for histopathology, culture, and rapid urease test) and non-invasive (urea breath tests, stool antigen test, and serological tests) methods. The diagnostic decisions are based on the prevalence of H. pylori infection and age-related gastric cancer incidence. For instance, non-invasive techniques are preferred mostly where gastric cancer incidence is low. In contrast, endoscopy is recommended for patients who have a high probability of getting gastric cancer, such as patients who are above 60 years of age or younger patients in a few Asia Pacific countries, or patients with a family history of gastric cancer or geographic regions with a high incidence of gastric cancer.
For instance, the Japanese Society for Helicobacter suggests to patients that the diagnosis of H. pylori infection should be performed using at least one invasive and non-invasive method. However, increased accuracy is obtained by using multiple diagnostic tests. Although these tests have high accuracy, the endoscopy-based diagnostic methods are not recommended for screening purposes, mainly due to their invasiveness, high cost, and unavailability. Thus, the growing awareness spread by governments for proper diagnosis has led to the advent of breakthrough techniques, expected to fuel the market's growth. Further, in 2020, RedHill Biopharma launched a nationwide H. pylori disease state educational field led by its sales force. The launch is intended to provide greater awareness to healthcare professionals regarding the risk of H. pylori infection and the growing resistance of H. pylori to standard care of antibiotics, leading to 25-40% failure of current therapies.
Market Overview
Asia Pacific is the fastest-growing market for Helicobacter pylori (H. pylori) non-invasive testing. The Asia Pacific Helicobacter pylori (H. pylori) non-invasive testing market is segmented into China, India, Japan, South Korea, Australia, and the Rest of Asia Pacific. Asia Pacific accounted for over 22.76% of the global Helicobacter pylori (H. pylori) non-invasive testing market in 2021 due to the rising geriatric population and high prevalence of H. pylori and gastric cancer cases. Therefore, the region holds huge potential for the market players to grow during the forecast period. China is a developing country in Asia Pacific with a well-established healthcare system and a fast-growing pharmaceutical industry. China is home to various medical, pharmaceutical, and biotechnology product manufacturing companies operating in the global market. These pharmaceuticals and biotechnology companies are involved in the development of various testing kits and devices for the dectection of Helicobacter pylori owing to the high prevalence of various H. pylori infections and gastric (stomach) cancer cases, which boosts the growth of the Helicobacter pylori non-invasive testing market.
The rising geriatric population is expected to offer vital growth opportunities for the Helicobacter pylori (H. pylori) non-invasive testing market in the coming years. However, the prevalence of Helicobacter pylori infection is significant in all ages; however, the geriatric population is likely to diagnose with gastric cancer and infections that can lead to serious illness and other medical emergencies. According to World Health Organization, in China, in 2019, there were ~254 million people of age 60 and older and ~176 million people of age 65 and over. It is estimated that ~402 million people (28% of the total population) in China will be over the age of 60 by 2040, which will create an opportunity for the use of various H. pylori testing kits, leading to the market growth in the coming years.
Strategic insights for the Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
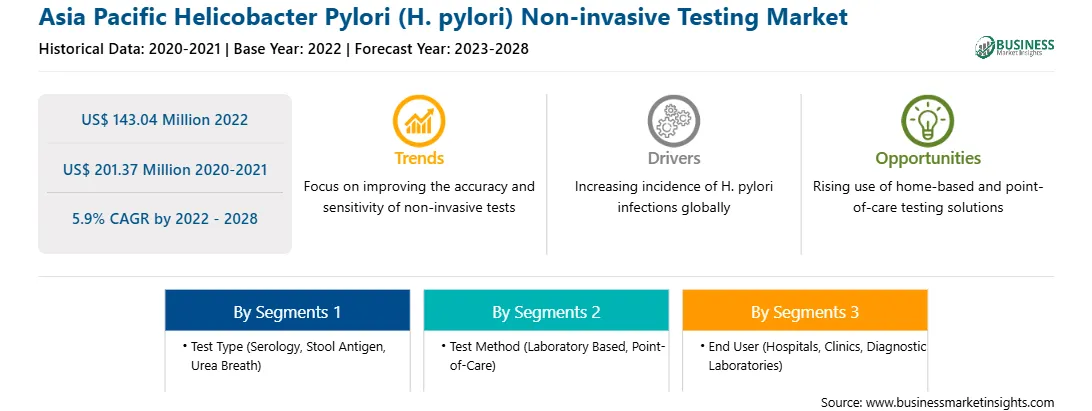
| Market size in 2022 | US$ 143.04 Million |
| Market Size by 2028 | US$ 201.37 Million |
| Global CAGR (2022 - 2028) | 5.9% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Test Type
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
The geographic scope of the Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing Market Segmentation
The Asia Pacific helicobacter pylori (H. pylori) non-invasive testing market is segmented on test type, test method, end user, and country.
Based on test type, the Asia Pacific Helicobacter pylori (H. pylori) non-invasive testing market is segmented into serology, stool antigen, and urea breathe. In 2022, the urea breath segment is expected to hold the largest share of the market. Based on test method, the Asia Pacific Helicobacter pylori (H. pylori) non-invasive testing market is bifurcated into laboratory based and point of care. The laboratory based segment is expected to hold a larger share of the market in 2022. Based on end user, the Asia Pacific Helicobacter pylori (H. pylori) non-invasive testing market is segmented into hospitals, clinics, and diagnostic laboratories. The diagnostic laboratories segment is expected to hold the largest share of the market in 2022. Based on country, the Asia Pacific Helicobacter pylori (H. pylori) non-invasive testing market is segmented into Australia, China, India, Japan, South Korea, the Rest of Asia Pacific. Further, China dominated the market in 2022. Abbott Laboratories; Bio-Rad Laboratories Inc.; CerTest Biotec; Coris BioConcept; DiaSorin S.p.A.; Meridian Bioscience Inc.; QuidelOrtho Corporation; Sekisui Diagnostics; Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd; and Thermo Fisher Scientific Inc. are the leading companies operating in the helicobacter pylori (H. pylori) non-invasive testing market in Asia Pacific.
The Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing Market is valued at US$ 143.04 Million in 2022, it is projected to reach US$ 201.37 Million by 2028.
As per our report Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing Market, the market size is valued at US$ 143.04 Million in 2022, projecting it to reach US$ 201.37 Million by 2028. This translates to a CAGR of approximately 5.9% during the forecast period.
The Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing Market report:
The Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Helicobacter Pylori (H. pylori) Non-invasive Testing Market value chain can benefit from the information contained in a comprehensive market report.