Enzyme replacement therapy administration typically requires 1–2 hours and is repeated monthly. Infusions are usually administered at infusion centers; however, under certain conditions, they can be administered at home by a nurse. Enzyme testing is usually the initial diagnostic test, but genetic analysis of gene mutations adds precision. For instance, small-molecule therapies, including substrate reduction and chaperone therapies, have also been developed and are approved for a few lysosomal storage diseases (LSDs). Additionally, companies are working to develop new therapies at the genomic level. For instance, SmartPharm is working to replace the administration of recombinant proteins by introducing the gene into the body to make the protein available over a long period. Successful gene-encoded therapeutics are expected to prolong the period between treatments. It could eliminate the infrastructure of infusion now required to deliver these proteins, thus freeing the patients from burdens related to current enzyme replacement therapy administration. Therefore, the trend of combining gene therapy with enzyme replacement therapy is expected to grow during the forecast period.
The enzyme replacement therapy market in Asia Pacific is segmented into Japan, China, India, Australia, South Korea, and the Rest of Asia Pacific. The APAC enzyme replacement therapy market in Japan is growing due to robust research and increasing ERT product approvals. For instance, in March 2022, Sanofi announced that the Japanese Ministry of Health, Labor, and Welfare (MHLW) granted marketing authorization for Xenpozyme (olipudase alfa) for the treatment of acid sphingomyelinase deficiency (ASMD). Also, Xenpozyme is the first and only approved therapy in Japan indicated to treat acid sphingomyelinase deficiency. Moreover, Japan's technologically advanced environment helps companies develop new products. For instance, in March 2021, JCR Pharmaceuticals announced the approval of IZCARGO (Pabinafusp Alfa) to treat Hunter syndrome. Thus, technological advancement and rising drug approvals are expected to fuel the enzyme replacement therapy market in Japan during the forecast period.

Strategic insights for the Asia Pacific Enzyme Replacement Therapy provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 2,415.74 Million |
| Market Size by 2028 | US$ 3,783.02 Million |
| Global CAGR (2022 - 2028) | 7.8% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Enzyme Type
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
The geographic scope of the Asia Pacific Enzyme Replacement Therapy refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
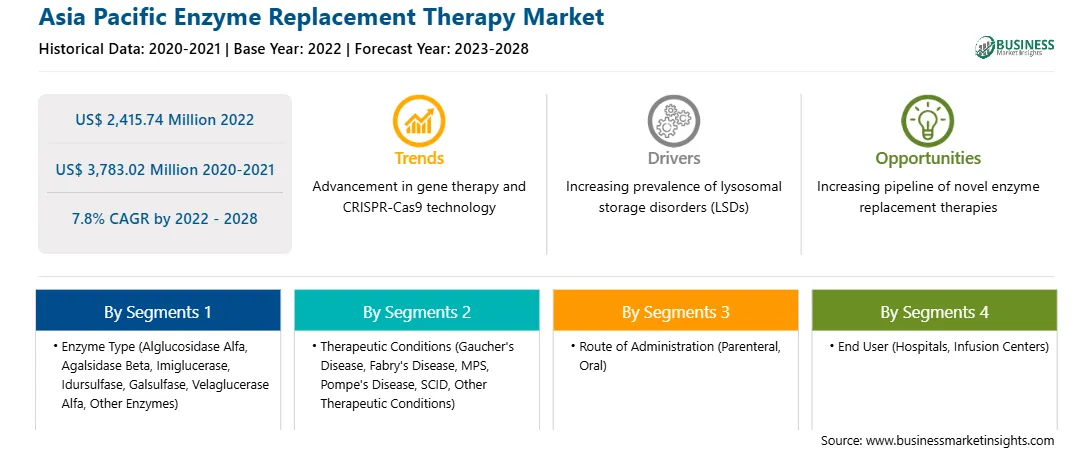
The Asia Pacific enzyme replacement therapy market is segmented into enzyme type, therapeutic conditions, route of administration, end user, and country.
By therapeutic conditions, the Asia Pacific enzyme replacement therapy market is segmented into Gaucher's disease, Fabry's disease, MPS, Pompe's disease, SCID, and other therapeutic conditions. The Gaucher's disease segment held the largest market share in 2022.
In terms of route of administration, the Asia Pacific enzyme replacement therapy market is bifurcated into parenteral and oral. The parenteral segment held a larger market share in 2022.
From end user point of reference, the Asia Pacific enzyme replacement therapy market is segmented into hospitals, infusion centers, and others. The hospitals segment held the largest market share in 2022.
Sanofi; BioMarine Pharmaceutical Inc; Takeda Pharmaceutical Company Limited; AbbVie Inc; Janssen Pharmaceutical (Johnson & Johnson Services, Inc.); Alexion Pharmaceutical, Inc. (AstaZeneca); Amicus Therapeutics; Recordati S.p.A; Recordati S.p.A; CHIESI farmaceutici S.p.A; and Pfizer Inc are the leading companies operating in the enzyme replacement therapy market in Asia Pacific.
The Asia Pacific Enzyme Replacement Therapy Market is valued at US$ 2,415.74 Million in 2022, it is projected to reach US$ 3,783.02 Million by 2028.
As per our report Asia Pacific Enzyme Replacement Therapy Market, the market size is valued at US$ 2,415.74 Million in 2022, projecting it to reach US$ 3,783.02 Million by 2028. This translates to a CAGR of approximately 7.8% during the forecast period.
The Asia Pacific Enzyme Replacement Therapy Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Enzyme Replacement Therapy Market report:
The Asia Pacific Enzyme Replacement Therapy Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Enzyme Replacement Therapy Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Enzyme Replacement Therapy Market value chain can benefit from the information contained in a comprehensive market report.