Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market
No. of Pages: 93 | Report Code: BMIRE00031353 | Category: Technology, Media and Telecommunications
No. of Pages: 93 | Report Code: BMIRE00031353 | Category: Technology, Media and Telecommunications
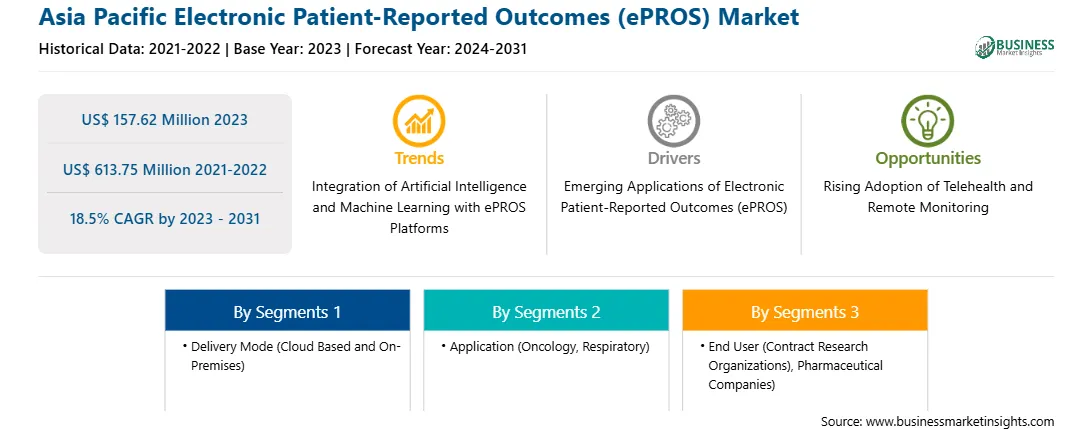
The Asia Pacific electronic patient-reported outcomes (ePROS) market was valued at US$ 157.62 million in 2023 and is expected to reach US$ 613.75 million by 2031; it is estimated to register a CAGR of 18.5% from 2023 to 2031.
Rising Adoption of Telehealth and Remote Monitoring Boosts Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market
The market for electronically reported patient outcomes (ePROs) is expected to experience lucrative opportunities with the rising adoption of telehealth and remote monitoring for clinical trials during the forecast period. Patients in remote or underserved areas can report real-time information from the comfort of their homes owing to telehealth and remote monitoring solutions, which increase accessibility of patient data. Health data can be continuously transmitted via remote monitoring devices, such as wearables and linked health systems. When coupled with ePRO systems, medical professionals receive detailed information regarding a patient's condition in real time, enabling more precise and regular interventions. For example, Vivalink's integrated acute remote patient monitoring solution provides continuous and real-time monitoring of patient vitals, including live ECG, as well as information about vitals and biometrics, EPRO/ECOA/surveys, centralized data services, remote data collection, and patient adherence. The efficacy of ePRO systems is expanded by telehealth and remote monitoring developments, establishing them as a crucial element of patient-centered care.
Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market Overview
China is expanding its support for clinical trials of drugs, which has resulted in the acceleration of new drug development. According to The Annual Report on the Progress of Clinical Trials for New Drug Registration in China, a total of 3,358 clinical trials of medications were registered in the country in 2021; 3,410 in 2022; and 4,300 in 2023. This indicates a constant growth in clinical trials. In addition, 500 biological products were approved, with oncology, dermatology, and endocrinology being key therapy areas. In addition, the country has emerged as an increasingly attractive R&D outsourcing destination for international pharmaceutical companies with an aim to reduce their product timeline and cost to the market. According to Clinical Trials Arena, the involvement of Western commercial companies in trial runs in the country has increased gradually from 100 trials per year in 2010 to ~350 trials in 2021. The surge in the number of clinical trials is creating a demand for ePROs to have real-time patient data and feedback during the trials, which also helps the manufacturer in the effective evaluation of the clinical trials. Moreover, increasing adoption of digital health technologies and supporting regulatory policies are expected to create ample opportunities for the electronic related patient outcomes (ePROS) market in the coming years.
Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market Revenue and Forecast to 2031 (US$ Million)
Strategic insights for the Asia Pacific Electronic Patient-Reported Outcomes (ePROS) provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Asia Pacific Electronic Patient-Reported Outcomes (ePROS) refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.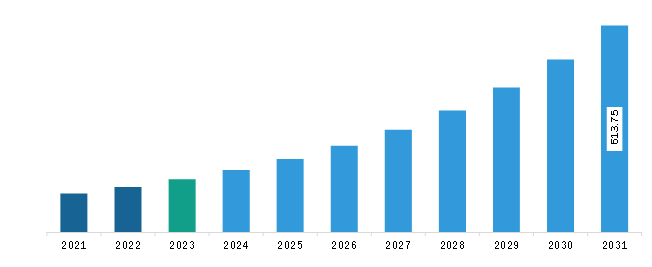
Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Strategic Insights
Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Report Scope
Report Attribute
Details
Market size in 2023
US$ 157.62 Million
Market Size by 2031
US$ 613.75 Million
Global CAGR (2023 - 2031)
18.5%
Historical Data
2021-2022
Forecast period
2024-2031
Segments Covered
By Delivery Mode
By Application
By End User
Regions and Countries Covered
Asia Pacific
Market leaders and key company profiles
Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Regional Insights
Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market Segmentation
The Asia Pacific electronic patient-reported outcomes (ePROS) market is categorized into delivery mode, application, end user, and country.
By delivery mode, the Asia Pacific electronic patient-reported outcomes (ePROS) market is bifurcated into cloud based and on-premises. The cloud based segment held a larger share of the Asia Pacific electronic patient-reported outcomes (ePROS) market share in 2023.
In terms of application, the Asia Pacific electronic patient-reported outcomes (ePROS) market is segmented into oncology, respiratory, and others. The oncology segment held the largest share of the Asia Pacific electronic patient-reported outcomes (ePROS) market share in 2023.
By end user, the Asia Pacific electronic patient-reported outcomes (ePROS) market is segmented into contract research organizations (CROs), pharmaceutical companies, and others. The pharmaceutical companies segment held the largest share of the Asia Pacific electronic patient-reported outcomes (ePROS) market share in 2023.
Based on country, the Asia Pacific electronic patient-reported outcomes (ePROS) market is segmented into Japan, China, India, South Korea, Australia, and the Rest of Asia Pacific. China segment held the largest share of Asia Pacific electronic patient-reported outcomes (ePROS) market in 2023.
Assistek, Buddy Healthcare Ltd Oy, Castor, Clinical Ink Inc, Crucial Data Solutions, Curebase, Medable Inc, Medidata Solutions, Medrio, OpenClinica LLC, PatientIQ, Signant Health, Veeva Systems Inc, and Y-Prime LLC are some of the leading companies operating in the Asia Pacific electronic patient-reported outcomes (ePROS) market.
The Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market is valued at US$ 157.62 Million in 2023, it is projected to reach US$ 613.75 Million by 2031.
As per our report Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market, the market size is valued at US$ 157.62 Million in 2023, projecting it to reach US$ 613.75 Million by 2031. This translates to a CAGR of approximately 18.5% during the forecast period.
The Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market report:
The Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Electronic Patient-Reported Outcomes (ePROS) Market value chain can benefit from the information contained in a comprehensive market report.