The clinical trial is an investigation study that defines whether a medical approach, therapy, or device is effective, safe, and useful for human applications. These studies help to find which therapeutic approaches experiment is best for certain diseases. Clinical trial supplies management is necessary for evading overproduction, oversupply, and inventory expiration. With the increasing costs of drug discovery, clinical trial supplies are obtaining more importance.
Strategic insights for the Asia Pacific Clinical Trials Supplies provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
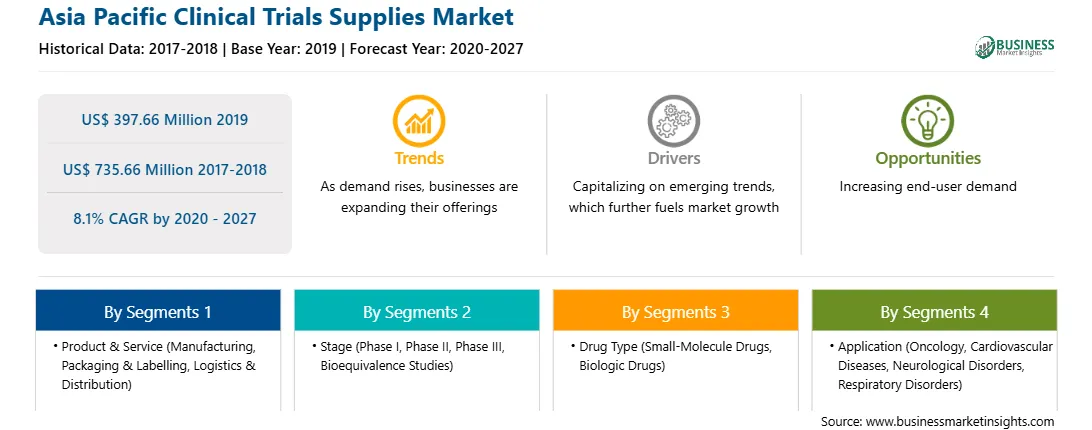
| Market size in 2019 | US$ 397.66 Million |
| Market Size by 2027 | US$ 735.66 Million |
| Global CAGR (2020 - 2027) | 8.1% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Product & Service
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
The geographic scope of the Asia Pacific Clinical Trials Supplies refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
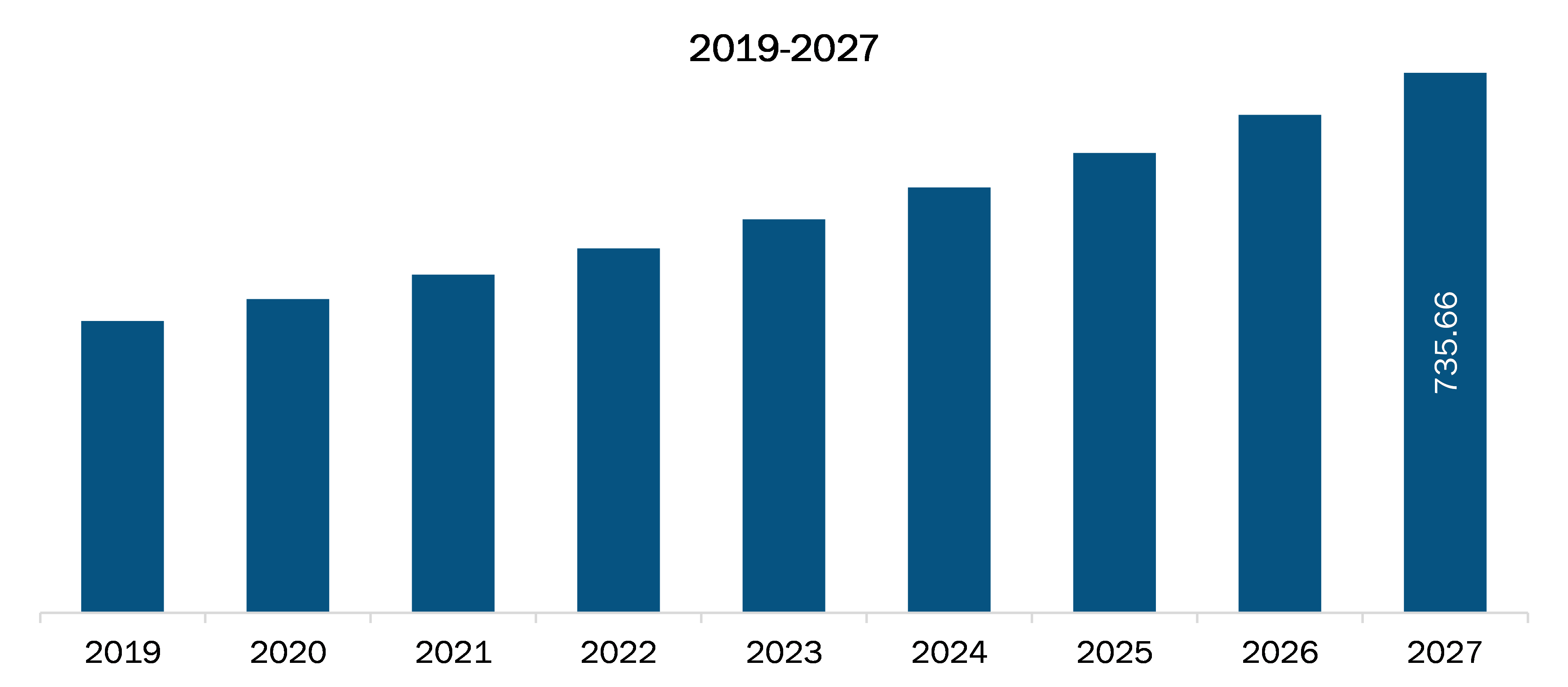
The Asia Pacific clinical trial supplies market is expected to reach US$ 735.66 Mn by 2027 from US$ 397.66 Mn in 2019; it is estimated to grow at a CAGR of 8.1% from 2020 to 2027. The growth of the market is attributed to some key driving factors such as increasing prevalence of chronic diseases, low cost of conducting a clinical trial compared to western region and increasing government initiatives to conduct clinical trials. However, challenges for clinical trials due to negative impact of COVID-19 pandemic is expected to restraint the growth of the market during the forecast years.
Clinical trial supplies management is necessary for evading overproduction, oversupply, and inventory expiration. With the increasing costs of drug discovery, clinical trial supplies are obtaining more importance. The growing healthcare sector in developing countries of Asia-Pacific creates better opportunities for the clinical trial supplies management market players to expand their business. The huge patient population in these countries is generating demand for more clinical trials. The increasing number of clinical trials conducted in Asia Pacific is ascribed to low operational costs, large patient recruitment potential, growth of contract research organizations (CROs), favorable regulatory environment, and better clinical trials capacity and quality. Increase in incidence of chronic diseases in Asia is a key factor that is likely to contributing to the surge in the number of clinical trials in Asia. Moreover, the clinical trials in Asia cost ~30–40% lower than the US and the EU, as the doctor visits, medical treatments, and procedures are less expensive in Asian countries. Also, higher cost of drug development process in developed countries will help propel market growth.
Since the surge of COVID-19 cases, the pharmaceutical industry is facing a decrease in production capabilities, resulting in drug shortages. Moreover, diversion of resources from drug development to coronavirus treatment is likely to hamper overall productivity of the drug development for a short period of span. Various pharmaceutical companies made decisions to postpone the ongoing clinical trials due to disrupted supply chains and threat of the virus. However, several contract research organizations based in Asia Pacific are engaged in a large number of clinical trials for different therapeutics. The outbreak has forced many CROs to close their manufacturing and development plants to avoid the increase in cases. Thus, the COVID-19 pandemic had a positive as well as negative impact on the Asia Pacific clinical trial supplies market.
In terms of product & service, the Asia Pacific clinical trial supplies market is segmented into manufacturing, packaging & labelling, and logistics & distribution. The logistics & distribution segment held the largest share of the market in 2019; the same segment is anticipated to register the highest CAGR in the market during the forecast period.
Based on stage, the Asia Pacific clinical trial supplies market is segmented into phase I, phase II, phase III, and bioequivalence studies. The phase III segment held the largest share of the market in 2019 and is estimated to register the highest CAGR in the market during the forecast period.
In terms of drug type, the Asia Pacific clinical trial supplies market is segmented into small-molecule drugs and biologic drugs. The small-molecule drugs segment held the largest share of the market in 2019, whereas the biologic drugs segment is estimated to register the highest CAGR in the market during the forecast period.
Based on application, the Asia Pacific clinical trial supplies market has been segmented into oncology, cardiovascular diseases, neurological disorders, respiratory disorders, and others. The oncology segment held the largest share of the market in 2019 and is estimated to register the highest CAGR during the forecast period.
A few of the primary and secondary sources associated with this report on the Asia Pacific clinical trial supplies market are the India Brand Equity Foundation (IBEF), National Institute for Health (NIH) and Association of Clinical Research Organizations (ACRO).
By Product & Service
By Stage
By Drug Type
By Application
The Asia Pacific Clinical Trials Supplies Market is valued at US$ 397.66 Million in 2019, it is projected to reach US$ 735.66 Million by 2027.
As per our report Asia Pacific Clinical Trials Supplies Market, the market size is valued at US$ 397.66 Million in 2019, projecting it to reach US$ 735.66 Million by 2027. This translates to a CAGR of approximately 8.1% during the forecast period.
The Asia Pacific Clinical Trials Supplies Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Clinical Trials Supplies Market report:
The Asia Pacific Clinical Trials Supplies Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Clinical Trials Supplies Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Clinical Trials Supplies Market value chain can benefit from the information contained in a comprehensive market report.