Adoption of Artificial Intelligence (AI) offers innovative ways to collect and manage clinical trial data and reduces dependency on manual operations. Thus, AI acts as a game changer for life science companies involved in the drug development process.
The Oncology Data Science team at AstraZeneca feeds these data into a system that utilizes AI and other statistical tools to generate novel hypotheses for oncology drug development. To transform the process of oncology data feed, the team is adopting complex datasets for accessibility, interoperability, and reusability of data as per "GOFAIR (Findability, Accessibility, Interoperability, and Reuse of digital assets)"—the set of principles. Such integration empowers data collection from specific clinical trials and projects to be accessible across the company's drug development teams in compliance with data protection laws.
AstraZeneca is collaborated with companies such as Tempus to leverage real-world data and represent patients globally. Such strategic partnerships are anticipated to offer crucial evidence about patient outcomes without revealing the identification of the clinical trial participants in the datasets.
Asia Pacific (APAC) is the fastest-growing regional market for global clinical trials and is segmented into China, Japan, India, South Korea, Australia, Indonesia, Thailand, Vietnam, and the Rest of Asia Pacific. Countries such as Australia, India, and South Korea are estimated to witness various growth opportunities due to the rising development in the healthcare sector. In addition, governments of these countries are increasing their efforts to provide clinical trials. Also, rise in the incidence of chronic diseases and growing awareness regarding clinical trials are likely to offer greater growth opportunities to the market players in the coming years.
Strategic insights for the Asia Pacific Clinical Trials provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market. The geographic scope of the Asia Pacific Clinical Trials refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.Asia Pacific Clinical Trials Strategic Insights
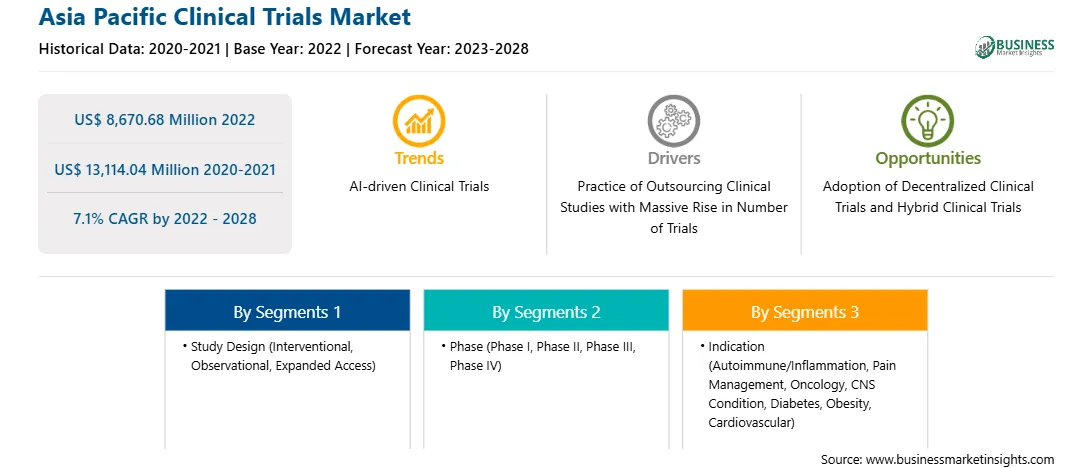
Asia Pacific Clinical Trials Report Scope
Report Attribute
Details
Market size in 2022
US$ 8,670.68 Million
Market Size by 2028
US$ 13,114.04 Million
Global CAGR (2022 - 2028)
7.1%
Historical Data
2020-2021
Forecast period
2023-2028
Segments Covered
By Study Design
By Phase
By Indication
Regions and Countries Covered
Asia-Pacific
Market leaders and key company profiles
Asia Pacific Clinical Trials Regional Insights
Asia Pacific Clinical Trials Market Segmentation
The Asia Pacific clinical trials market is segmented into phase, study design, indication, and country.
Based on phase, the Asia Pacific clinical trials market is segmented into phase I, phase II, phase III, and phase IV. The phase III segment registered the largest Asia Pacific clinical trials market share in 2022.
Based on study design, the Asia Pacific clinical trials market is segmented into interventional, observational, and expanded access. The interventional segment held the largest Asia Pacific clinical trials market share in 2022.
Based on indication, the Asia Pacific clinical trials market is segmented into autoimmune/inflammation, pain management, oncology, CNS condition, diabetes, obesity, cardiovascular, and others. The oncology segment held the largest Asia Pacific clinical trials market share in 2022.
Based on country, the Asia Pacific clinical trials market has been categorized into China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific. China dominated the Asia Pacific clinical trials market in 2022.
Charles River Laboratories International Inc, ICON Plc, IQVIA Holdings Inc, Laboratory Corp of America Holdings, Parexel International Corp, SGS SA, SIRO Clinpharm Pvt Ltd, Syneos Health Inc, Thermo Fisher Scientific Inc, and WuXi AppTec Co Ltd. are some of the leading companies operating in the Asia Pacific clinical trials market.
The Asia Pacific Clinical Trials Market is valued at US$ 8,670.68 Million in 2022, it is projected to reach US$ 13,114.04 Million by 2028.
As per our report Asia Pacific Clinical Trials Market, the market size is valued at US$ 8,670.68 Million in 2022, projecting it to reach US$ 13,114.04 Million by 2028. This translates to a CAGR of approximately 7.1% during the forecast period.
The Asia Pacific Clinical Trials Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Clinical Trials Market report:
The Asia Pacific Clinical Trials Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Clinical Trials Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Clinical Trials Market value chain can benefit from the information contained in a comprehensive market report.