Biologicals represent promising new therapies for previously incurable diseases and are becoming highly important in the pharmaceuticals market. Patents on originator biologicals are expected to expire in the coming years. The patent expiration and other intellectual property rights for originator biologicals will create a need to introduce new biosimilars in the future. As a result, competition among market players will surge in the industry in the coming years. Thus, the patent expiry of blockbuster biologics is expected to create lucrative opportunities for the biosimilar market during the forecast period.
According to Generics and Biosimilars Initiative (GaBI), in 2021, the National Medical Products Administration (NMPA) has approved 13 copy biologicals, within the product classes of monoclonal antibody and tumour necrosis factor (TNF)-inhibitor, for use in China.
In February 2019, China’s first official biosimilar was approved. The rituximab biosimilar HLX01 was developed by Shanghai Henlius Biopharmaceutical for the treatment of Non-Hodgkin’s Lymphoma (NHL). Three more biosimilars were approved in China in 2019, and seven biosimilars were approved in 2020, at an increase of 75% between 2019 and 2020. A bevacizumab biosimilar by Luye Pharma Group Ltd was approved in May 2021 for non-small cell lung cancer, and an infliximab biosimilar by Mabpharm Ltd was approved in July 2021 for ankylosing spondylitis.
Thus, the Chinese biosimilars will continue to surge in the future, with 11 biosimilars in pre-registration awaiting NMPA approval and approximately 100 biosimilars in development. Therefore, China's strong biosimilar pipeline and the NMPA's latest regulation changes will foster growth in China's biosimilar market.
Strategic insights for the Asia Pacific Biosimilars provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
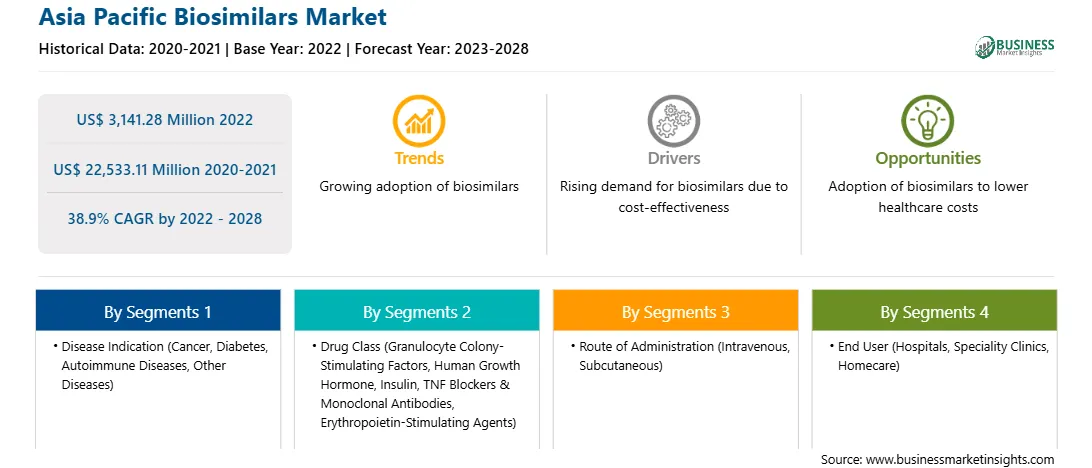
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 3,141.28 Million |
| Market Size by 2028 | US$ 22,533.11 Million |
| Global CAGR (2022 - 2028) | 38.9% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Disease Indication
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
The geographic scope of the Asia Pacific Biosimilars refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
The Asia Pacific biosimilars market is segmented into disease indication, drug class, route of administration, end user, and country.
Based on disease indication, the biosimilars market is segmented into cancer, diabetes, autoimmune diseases, and other disease indications. The cancer segment held the largest market share in 2022.
The biosimilars market, based on drug class, is segmented into granulocyte colony-stimulating stimulating factors, human growth hormone, insulin, TNF blockers & monoclonal antibodies, erythropoietin-stimulating stimulating agents, and others. The granulocyte colony-stimulating factors segment accounted for the largest share of the market in 2022.
Based on route of administration, the biosimilar market is segmented into intravenous, subcutaneous, and others. The intravenous segment accounted for the largest share of the market in 2022.
The biosimilars market, based on end user, is segmented into hospitals, specialty clinics, homecare, and others. The hospitals segment accounted for the largest share of the market in 2022.
Based on country, the Asia Pacific biosimilars market is segmented into Australia, China, India, Japan, South Korea, and the Rest of Asia Pacific. China dominated the market in 2022.
Amgen Inc; Sanofi SA; Biocon Ltd; Eli Lilly and Co; Sandoz AG; Teva Pharmaceutical Industries Ltd; Pfizer Inc; and Dr. Reddy's Laboratories Ltd; Celltrion Inc; Samsung Bioepis Co Ltd are the leading companies operating in the Asia Pacific biosimilars market.
The Asia Pacific Biosimilars Market is valued at US$ 3,141.28 Million in 2022, it is projected to reach US$ 22,533.11 Million by 2028.
As per our report Asia Pacific Biosimilars Market, the market size is valued at US$ 3,141.28 Million in 2022, projecting it to reach US$ 22,533.11 Million by 2028. This translates to a CAGR of approximately 38.9% during the forecast period.
The Asia Pacific Biosimilars Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Biosimilars Market report:
The Asia Pacific Biosimilars Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Biosimilars Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Biosimilars Market value chain can benefit from the information contained in a comprehensive market report.