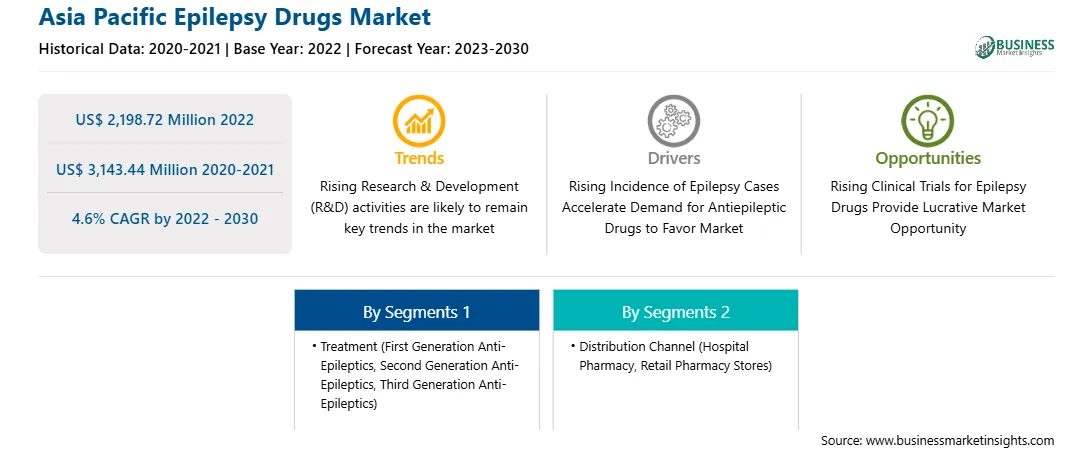
Asia Pacific Epilepsy Drugs Market
No. of Pages: 71 | Report Code: BMIRE00029811 | Category: Life Sciences
No. of Pages: 71 | Report Code: BMIRE00029811 | Category: Life Sciences
Many patents expiries are anticipated in the epilepsy drugs market in the upcoming years. It is likely to increase the penetration of generic products to cut down healthcare expenses. This will generate additional opportunities for other market players. Below mentioned are some drugs that are nearing patent expiration in the next couple of years.
Company | Patent | Patent Expiration Date | Ingredient | Treatment |
Sumitomo Pharma Co | US9206135 | April 21, 2026 | ESLICARBAZEPINE ACETATE | Partial-onset Seizures Epilepsy |
GlaxoSmithKline | US7919115 | January 4, 2029 | LAMOTRIGINE | Epilepsy |
Ucb Inc | USRE38551 | March 17, 2022 | Lacosamide | Epilepsy and Partial-onset Seizures |
SK Biopharmaceuticals | US7598279 | October 30, 2027 | CENOBAMATE | Partial Epilepsies |
SUPERNUS PHARMS | US8877248 | November, 2027 | Sustained-release formulations of Topiramate | Epilepsy |
SUPERNUS PHARMS | US9555004 | November, 2027 | Sustained-release formulations of Topiramate | Epilepsy |
SUPERNUS PHARMS | US10314790 | November, 2027 | Sustained-release formulations of Topiramate | Epilepsy |
SUPERNUS PHARMS | US8663683 | November, 2027 | Sustained-release formulations of Topiramate | Epilepsy |
SUPERNUS PHARMS | US9622983 | November, 2027 | Sustained-release formulations of Topiramate | Epilepsy |
SUPERNUS PHARMS | US8298580 | November, 2027 | Sustained-release formulations of Topiramate | Epilepsy |
SUPERNUS PHARMS | US8992989 | November, 2027 | Sustained-release formulations of Topiramate | Epilepsy |
SUPERNUS PHARMS | US8889191 | November, 2027 | Sustained-release formulations of Topiramate | Epilepsy |
SUPERNUS PHARMS | US9549940 | November, 2027 | Sustained-release formulations of Topiramate | Epilepsy |
SUPERNUS PHARMS | US8298576 | April, 2028 | Sustained-release formulations of Topiramate | Epilepsy |
The Asia Pacific epilepsy drugs market is analysed based on China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific. According to the World Health Organization (WHO) report, epilepsy is more common in developing countries than in developed countries, accounting for a prevalence of 6.1% and 5.0%, respectively. China is the most populated country in the world and the one with the most patients with neurological disorders. According to the Centers of Disease Control and Prevention, in China, the prevalence rate of active epilepsy is 0.48% to 8.5%; there are approximately 9 million people with epilepsy in Mainland China, two-thirds of whom are children. ~30% of patients with epilepsy are not capable of controlling their seizures with the AEDs that are currently available in the market; thus, this disease has significant unmet medical needs.
Moreover, the drug approvals in the country are propelling the market growth. For instance, in August 2021, Eisai Co., Ltd. obtained two additional approvals of the antiepileptic drug (AED) Fycompa, as “a monotherapy for partial-onset seizures” and “an adjunctive treatment / a monotherapy for a pediatric indication for partial onset seizures in patients with epilepsy four years of age and older” in China from the National Medical Products Administration.
The Asia Pacific epilepsy drugs market is segmented into treatment, distribution channel, and country.
Based on treatment, the Asia Pacific epilepsy drugs market is classified into first generation anti-epileptics, second generation anti-epileptics, and third generation anti-epileptics. The third-generation anti-epileptics segment held the largest share in 2022.
In terms of distribution channel, the Asia Pacific epilepsy drugs market is categorized into hospital pharmacy, retail pharmacy stores, and others. The hospital pharmacy segment held the largest share in 2022.
Based on country, the Asia Pacific epilepsy drugs market is segmented China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific. China dominated the Asia Pacific epilepsy drugs market in 2022.
Abbott Laboratories, Alkem Laboratories Ltd, GSK Plc, Novartis AG, Pfizer Inc, Sanofi SA, and Teva Pharmaceutical Industries Ltd are some of the leading companies operating in the Asia Pacific epilepsy drugs market.
Strategic insights for the Asia Pacific Epilepsy Drugs provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 2,198.72 Million |
| Market Size by 2030 | US$ 3,143.44 Million |
| Global CAGR (2022 - 2030) | 4.6% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Treatment
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
|
The geographic scope of the Asia Pacific Epilepsy Drugs refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
1. Abbott Laboratories
2. Alkem Laboratories Ltd
3. GSK Plc
4. Novartis AG
5. Pfizer Inc
6. Sanofi SA
7. Teva Pharmaceutical Industries Ltd
The Asia Pacific Epilepsy Drugs Market is valued at US$ 2,198.72 Million in 2022, it is projected to reach US$ 3,143.44 Million by 2030.
As per our report Asia Pacific Epilepsy Drugs Market, the market size is valued at US$ 2,198.72 Million in 2022, projecting it to reach US$ 3,143.44 Million by 2030. This translates to a CAGR of approximately 4.6% during the forecast period.
The Asia Pacific Epilepsy Drugs Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the Asia Pacific Epilepsy Drugs Market report:
The Asia Pacific Epilepsy Drugs Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The Asia Pacific Epilepsy Drugs Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the Asia Pacific Epilepsy Drugs Market value chain can benefit from the information contained in a comprehensive market report.