Report : Asia Pacific Vaccine Adjuvants Market Forecast to 2030 – Regional Analysis – by Adjuvant Class (Mineral Salt Adjuvant, Emulsion Adjuvant, Liposome Adjuvant, and Others) and Type (Human Vaccine Adjuvant and Veterinary Vaccine Adjuvant)
At 14.6% CAGR, Asia Pacific Vaccine Adjuvants Market is Projected to be Worth US$ 1,546.43 Million by 2030, says Business Market Insights
According to Business Market Insights’ research, the Asia Pacific vaccine adjuvants market was valued at US$ 518.07 million in 2022 and is expected to reach US$ 1,546.43 million by 2030, registering a CAGR of 14.6% from 2022 to 2030. Approvals of veterinary vaccine adjuvants and rising number of infectious disease outbreaks and pandemic are among the critical factors attributed to drive the Asia Pacific vaccine adjuvants market growth.
Vaccinating animals against common diseases remains the most successful method worldwide to prevent financial and other losses from infectious diseases in animal farming. Regulatory approvals for veterinary vaccine adjuvants are increasing, with manufacturers' growing focus on developing vaccines with greater efficacy and stability. Montanide is one of the known veterinary vaccine adjuvants. The Montanide adjuvant range is based on three core technologies—emulsions, micro-emulsions, and polymers. Below is the list of adjuvants used in commercial vaccines by animal species in 2022:
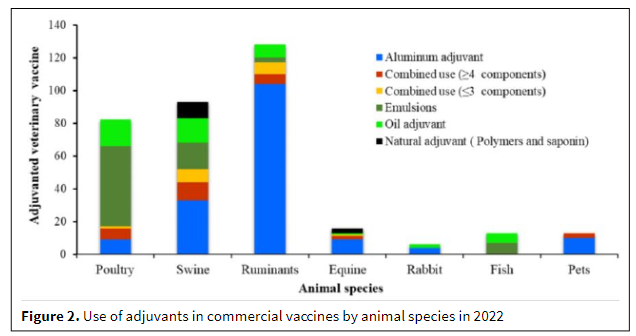
Source: Journal of Pharmacy & Pharmacognosy Research
Below is a list of approved veterinary vaccine adjuvants in India from January 2020 to September 2022.
Veterinary Vaccines Adjuvants for Veterinary Infections | ||
Company Name | Product | Disease Indication |
Boehringer Ingelheim | Inactivated Vaccine in Oil Adjuvant | Newcastle Disease, Disease Bronchitis, Infectious Bursal Disease, and Avial Viral Arthritis |
Virbac Animal Health Pvt. Ltd. | Inactivated Vaccine in Oil Adjuvant | Newcastle Disease and Ranikhet Disease |
Virbac Animal Health Pvt. Ltd. | Inactivated Vaccine in Oil Adjuvant | Newcastle Disease, Ranikhet Disease, Avian infectious Bronchitis, and Combined Newcastle Disease |
Source: CDSCO
Therefore, an upsurge in adjuvant approvals for veterinary vaccines accelerates the overall market growth of vaccine adjuvants.
On the contrary, product recalls and adverse effects hamper the growth of Asia Pacific vaccine adjuvants market.
Based adjuvant class, the Asia Pacific vaccine adjuvants market is segmented into mineral salt adjuvant, emulsion adjuvant, liposome adjuvant, and others. The mineral salt adjuvant segment held 40.0% share of Asia Pacific vaccine adjuvants market share in 2022, amassing US$ 207.20 million. It is projected to garner US$ 590.19 million by 2030 to register 14.0% CAGR during 2022–2030.
By type, the Asia Pacific vaccine adjuvants market is bifurcated into human vaccine adjuvant and veterinary vaccine adjuvant. The human vaccine adjuvant segment held 63.7% share of Asia Pacific vaccine adjuvants market in 2022, amassing US$ 330.19 million. It is anticipated to garner US$ 1,008.52 million by 2030 to expand at 15.0% CAGR during 2022–2030.
By country, the Asia Pacific vaccine adjuvants market is segmented into China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific. China segment held 27.2% share of Asia Pacific vaccine adjuvants market in 2022, amassing US$ 141.02 million. It is anticipated to garner US$ 435.78 million by 2030 to expand at 15.1% CAGR during 2022–2030
Key players operating in the Asia Pacific vaccine adjuvants market are Croda International Plc; CSL Ltd; GSK Plc; Hawaii Biotech Inc; InvivoGen SAS; Novavax Inc; Phibro Animal Health Corp; and SPI Pharma Inc, among others.
- October, 2023; SPI Pharma Inc and Q-Vant Biosciences Inc announced a partnership that combines Q-Vant's leadership in sustainable saponin extraction technology with SPI's global reach and servicing expertise in the pharmaceutical industry. The arrangement includes investment in expanding Q-Vant’s proprietary 100% sustainable Q-SAP technology and an exclusive commercial agreement to accelerate the global adoption of their saponin adjuvants for veterinary and human vaccine formulations.
Contact Us
Contact Person: Sameer Joshi
Phone: +1-646-491-9876
Email Id: sales@businessmarketinsights.com
