到2030年北美脊柱融合装置市场预测——区域分析——按产品类型(胸腰椎装置、颈椎固定装置和椎间融合装置)、手术类型(开放脊柱手术和微创脊柱手术)、疾病适应症(退行性椎间盘、创伤和骨折、复杂畸形等)和最终用户(医院、专科诊所等)
No. of Pages: 109 | Report Code: BMIRE00029460 | Category: Life Sciences
No. of Pages: 109 | Report Code: BMIRE00029460 | Category: Life Sciences
脊柱器械公司广泛采用创新、分拆以及并购发展或战略。这些战略有助于标志着北美脊柱融合装置市场在 2022 年实现指数级增长。以下是这些公司在 2022 年取得的一些进展实例。
&bull ; 2022 年 8 月,凯鹏华盈设备实验室宣布使用其新型 KG2 Surge flow-thru 椎间系统成功完成了首次手术。 2022年7月19日,美国纽约布鲁克林医院中心实施单节段经椎间孔腰椎椎间融合术(TLIF)手术。 Kleiner Device Labs 声称,新型 KG2 Surge flow-thru 椎间系统提供的骨移植材料量是传统脊柱手术方法的三倍。该公司还声称,他们的 KG2 Surge flow-thru 椎间系统是一种单患者使用的骨移植输送工具,与 3D 打印的钛 I-Beam 融合植入物相结合。此外,KG2 Surge 流通式椎间系统将把多步骤、多器械通过练习减少为当前脊柱融合手术的单一插入过程。
• 2022 年 3 月,Zimmer Biomet Holdings, Inc. 宣布完全分拆 ZimVie。 ZimVie 将继续专注于脊柱和牙科市场的关键增长战略。该公司将开发产品,包括脊柱融合植入物、手术工具、骨移植物、植入物、非融合替代品和数字护理技术。由于分拆,ZimVie 将作为在纳斯达克注册的独立实体运营,代码为“ZIMV”。因此,预计 ZimVie 将通过保持良好的市场份额来显着增强市场。
• 2022 年 10 月,Spineart 推出了新型微创脊柱手术 (MISS) 系统 PERLA TL MIS。该公司的 MISS 系统是一种在全球范围内销售的胸腰椎后路固定系统。 PERLA TL MIS 的推出预计将表明该公司致力于改变脊柱手术,使其最终用户受益:外科医生、患者和医院。此类产品的推出可能会在未来推动市场增长。
脊柱行业的发展趋势仍在持续,并见证了战略改进,推动了北美脊柱融合装置市场的增长。< /p>
• 2023年7月,Xtant Medical Holdings, Inc.宣布获得Surgalign Holdings, Inc.与脊柱固定及国内外生物制剂相关的部分资产和负债。 Xtant Medical Holdings, Inc. 致力于开发脊柱植入系统,以促进复杂脊柱畸形退行过程中的脊柱融合。因此,脊柱设备公司在脊柱技术方面的这种发展正在促进市场增长。
DePuy Synthes、Stryker、Aurora Spine 和 Alevio Spine 是北美脊柱融合领域的主要参与者美国的设备市场。这些参与者推动的产品开发和发布有利于市场增长。经美国食品和药物管理局 (FDA) 批准的技术先进的脊柱融合装置在美国得到广泛采用。以下是 FDA 最近批准的脊柱融合装置列表:
• 2023 年 5 月,CTL Amedica 获得 FDA 510(k) 许可,允许 NITRO 椎间融合笼系统商业化,该系统专门由生物材料氮化硅融合而成。氮化硅材料与所有成像模式兼容;它具有独特的抑菌特性并提供无伪影成像。
• 2023 年 1 月,Alevio Spine 获得了 SI-Cure SI 关节融合系统附加适应症的 510 (K) 许可。扩大的适应症包括对骨骼成熟的患者进行骶髂融合,作为腰椎或胸腰椎融合的一部分进行骶骨盆固定。
• 2022 年 6 月,美国 FDA 批准了 Aurora Spine 的 DEXA SOLO-L 前路腰椎椎间融合器 (ALIF) 的 510K 许可。基于 DEXA 技术平台,3D 打印独立设备专为前侧和侧向腰椎椎间融合 (ALIF & LLIF) 手术而设计。
与年龄相关的磨损会引发腰痛的患病率美国老年人群中的 LBP 反过来又刺激了对脊柱融合装置的需求。根据 2022 年国家卫生服务部门的数据,美国 LBP 的终生发病率为 60-90%,年发病率为 5%。消息来源还指出,每年有 14.3% 的新患者因 LBP 就诊,约 1300 万人因慢性 LBP 就医。
北美脊柱融合装置市场根据产品类型、手术类型、疾病适应症、结束时间进行细分
根据产品类型,北美脊柱融合装置市场分为胸腰椎装置、颈椎固定装置和椎间融合装置。到2022年,胸腰椎器械市场占据最大份额。
按手术类型划分,北美脊柱融合器械市场分为开放脊柱手术和微创脊柱手术。 2022 年,开放式脊柱手术细分市场占据最大份额。
按照疾病适应症,北美脊柱融合装置市场分为退行性椎间盘、外伤和骨折、复杂畸形等。退行性椎间盘细分市场在 2022 年占据最大份额。
就最终用户而言,北美脊柱融合装置市场分为两类: 医院、专科诊所等。 2022 年,医院细分市场占据最大份额。
根据国家/地区,北美脊柱融合装置市场分为美国、加拿大和墨西哥。 2022 年,美国将主导北美脊柱融合装置市场。
ATEC Spine Inc、B. Braun SE、Centinel Spine LLC、DePuy Synthes Inc、Globus Medical Inc、Medtronic Plc、NuVasive Inc、Orthofix Medical Inc、Stryker Corp 和 ZimVie Inc 是北美脊柱融合设备市场上的一些领先公司。
Strategic insights for North America Spinal Fusion Devices involve closely monitoring industry trends, consumer behaviours, and competitor actions to identify opportunities for growth. By leveraging data analytics, businesses can anticipate market shifts and make informed decisions that align with evolving customer needs. Understanding these dynamics helps companies adjust their strategies proactively, enhance customer engagement, and strengthen their competitive edge. Building strong relationships with stakeholders and staying agile in response to changes ensures long-term success in any market.
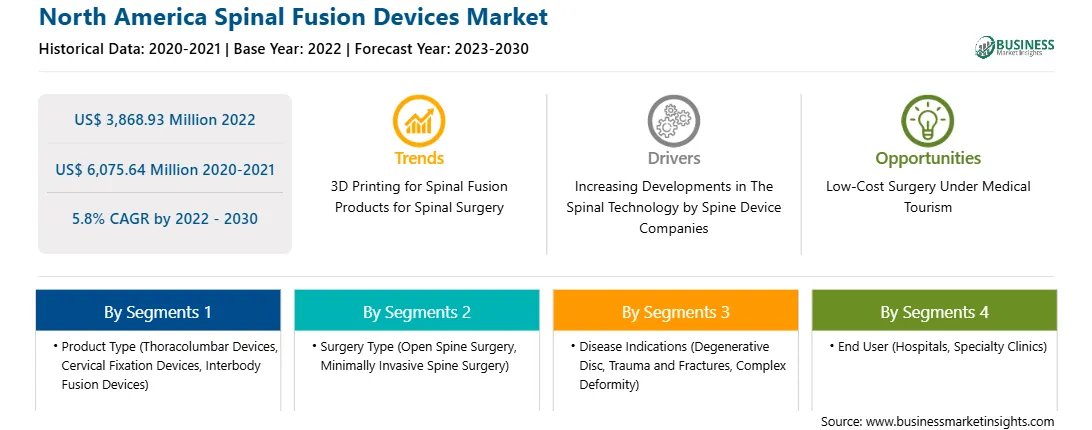
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 3,868.93 Million |
| Market Size by 2030 | US$ 6,075.64 Million |
| Global CAGR (2022 - 2030) | 5.8% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By 产品类型
|
| Regions and Countries Covered | 北美
|
| Market leaders and key company profiles |
The regional scope of North America Spinal Fusion Devices refers to the geographical area in which a business operates and competes. Understanding regional nuances, such as local consumer preferences, economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved regions or adapting their offerings to meet regional demands. A clear regional focus allows for more effective resource allocation, targeted marketing, and better positioning against local competitors, ultimately driving growth in those specific areas.
The North America Spinal Fusion Devices Market is valued at US$ 3,868.93 Million in 2022, it is projected to reach US$ 6,075.64 Million by 2030.
As per our report North America Spinal Fusion Devices Market, the market size is valued at US$ 3,868.93 Million in 2022, projecting it to reach US$ 6,075.64 Million by 2030. This translates to a CAGR of approximately 5.8% during the forecast period.
The North America Spinal Fusion Devices Market report typically cover these key segments-
The historic period, base year, and forecast period can vary slightly depending on the specific market research report. However, for the North America Spinal Fusion Devices Market report:
The North America Spinal Fusion Devices Market is populated by several key players, each contributing to its growth and innovation. Some of the major players include:
The North America Spinal Fusion Devices Market report is valuable for diverse stakeholders, including:
Essentially, anyone involved in or considering involvement in the North America Spinal Fusion Devices Market value chain can benefit from the information contained in a comprehensive market report.