North America Healthcare Regulatory Affairs Outsourcing Market Forecast to 2028 - COVID-19 Impact and Regional Analysis By Service Type (Regulatory & Scientific Strategy Development, Medical & Scientific Writing, eCTD & e-Submissions, Data Management Services, Life Cycle Management Services, Pharmacovigilance, Chemistry Manufacturing & Controls (CMC) Services, Regulatory Labelling, and Regulatory Artwork Services) and End User (Pharmaceutical Companies, Biotechnology Companies, and Medical Devices Companies)
Market Introduction
The North America healthcare regulatory affairs outsourcing market is categorized into the US, Canada, and Mexico. North America is likely to capture significant share of the global market in 2021. The key factors that are driving the growth of the market are rising number of patent expirations along with growing costs of research and development activities. However, during the forecast period the market is likely to get restrained by the factors such as high fluctuations in price along with hidden expenses in the regulatory services delivered by diverse Clinical Research Organizations. The US is the largest market for healthcare regulatory affairs outsourcing at a global level. The market's growth is attributed to the enormous number of R&D activities in the field of drug discovery carried out in the country. The U.S. healthcare industry’s effort to minimize costs without compromising on quality of services is the major factor for the outsourcing of healthcare and medical services to other countries. The rigorous regulations imposed by the Health Insurance Portability and Accountability Act (HIPAA) of 1996 towards the establishment of national standards for electronic health care transactions and national identifiers for providers, health insurance plans, and employers has boosted the market growth. The standards and regulations are compulsory and require infrastructure which hospitals lack, thus making outsourcing unavoidable, and driving the growth of the U.S. healthcare outsourcing market. The rapid developments in the market and entry of global players into the market have resulted in partnerships, mergers & acquisitions, and joint ventures in the market. Moreover, the medical service vendors are mostly involved in making strategic partnerships with hospitals to provide outsourcing solutions. Rising regulatory pressure on healthcare companies is the major factor driving the growth of the North America healthcare regulatory affairs outsourcing market.
COVID-19 virus outbreak was first observed in December 2019 in Wuhan (China), and it has spread to ~100 countries across the world, with the World Health Organization (WHO) stating it as a public health emergency. The global impacts of COVID-19 are being felt across several markets. Although the healthcare sector had witnessed SARS, H1N1, and other outbreaks in the last few years, the severity of the COVID-19 has made the situation more complicated due to its mode of transmission. North America witnessed growing number of COVID-19 cases since its outbreak. For instance, according to Worldometer, the number of cases reached to 34,434,803 million with 617,875 deaths reported in the United States as of 23rd June 2021. Similar impact was noticed in Mexico and Canada. North America is one of the most important regions for the adoption and growth of new technologies due to flattering government policies to boost innovation, the presence of a huge industrial base, and high purchasing power, mainly in developed countries such as the US and Canada. Hence, any impact on the growth of industries is expected to affect the region's economic growth negatively. Presently, the US is the world’s worst-affected country due to the COVID-19 outbreak, with 28,659,480 confirmed cases and 520,751 deaths as per the WHO. The US is one of the eminent markets for healthcare outsourcing services. The factory and business shutdowns across the US, Canada, and Mexico negatively impacted various industries in 2020. However, COVID-19 has placed many regulatory and outsourcing teams under pressure but, it also has had a positive impact on the bio/pharmaceutical outsourcing industry, wherein the demand for R&D activity is increasing leading to a rise in regulatory affairs assistance. This rising demand has caused various CROs to focus on their outsourcing and other operations.
Get more information on this report : 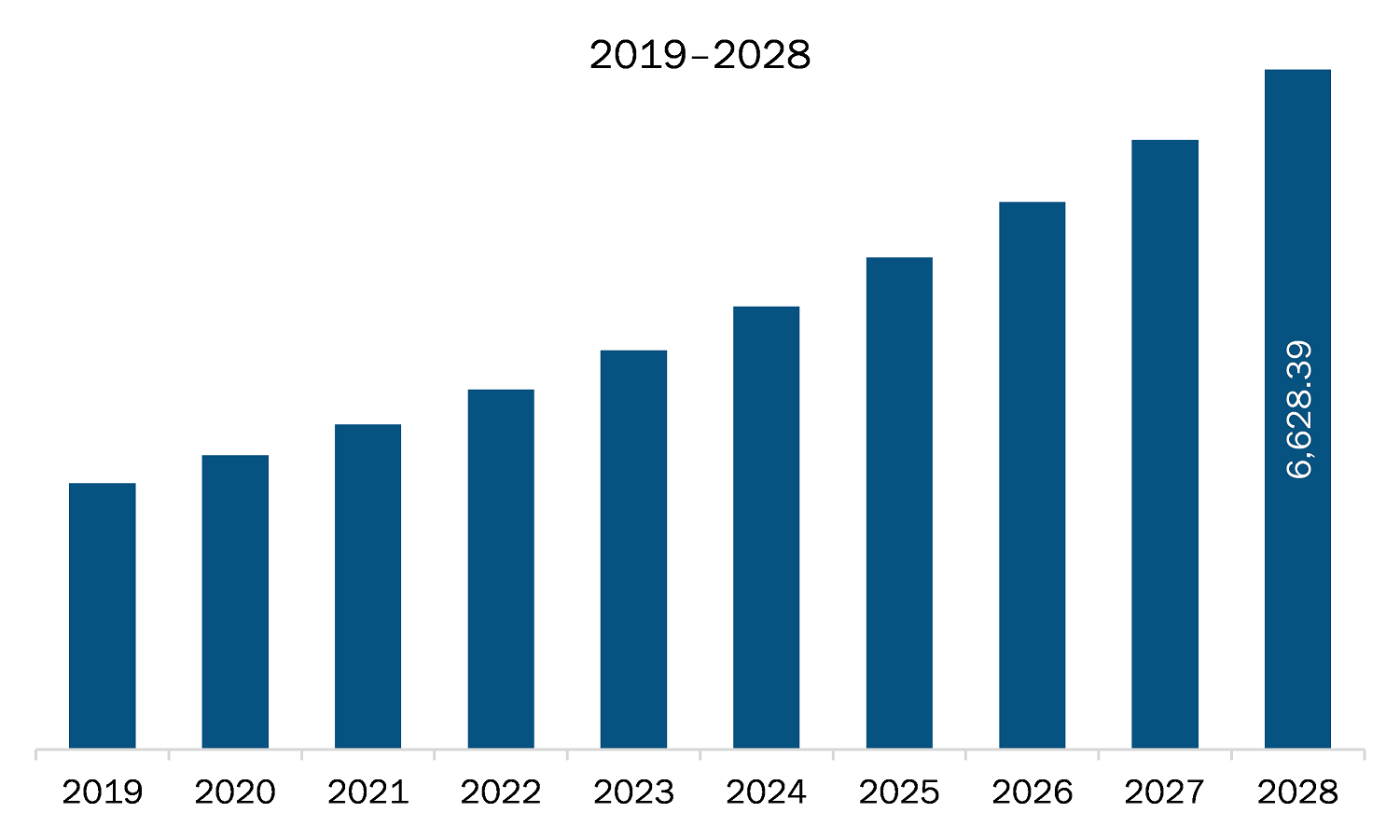
Market Overview and Dynamics
The healthcare regulatory affairs outsourcing market in North America is expected to grow from US$ 3,171.78 million in 2021 to US$ 6,628.39 million by 2028; it is estimated to grow at a CAGR of 11.1% from 2021 to 2028. The development of blockbuster therapies such as targeted gene therapies, specialty drugs, and precision medicine that help treat specific diseases and disorders has been a major focus in the healthcare sector for a long period. A few of these therapies are also being combined with medical devices to enhance the quality of drug delivery, dose, and patient monitoring or adherence, which is expected to add to the complexity of the related regulatory strategies and difficulties in their way to market. Thus, developments in emerging segments in healthcare sectors such as specialty therapies, orphan drugs, and personalized medicines are expected to offer significant growth opportunities to healthcare regulatory affairs outsourcing market players during the forecast period.
Key Market Segments
The North America healthcare regulatory affairs outsourcing market has been segmented based on service type, end user, and country. On the basis of service type, the North America healthcare regulatory affairs outsourcing market is segmented into medical & scientific writing, pharmacovigilance, data management services, life cycle management services, eCTD and e-Submissions, regulatory and scientific strategy development, chemistry manufacturing and controls (CMC) services, regulatory labelling, and regulatory artwork services. The medical & scientific writing segment dominated the market in 2020 and pharmacovigilance segment is expected to be the fastest growing during the forecast period. Based on end user, the market is segmented into pharmaceutical companies, biotechnology companies, and medical devices companies. The pharmaceutical companies segment dominated the market in 2020 and is expected to be the fastest growing during the forecast period. Likewise, the medical devices companies segmented is categorized into medical device materials & biomaterials, medical device, biomarkers and in vitro diagnostics (IVD), medical device software (SaMD), medical device electromechanics, medical device substance-based, and medical device of combination product.
Major Sources and Companies Listed
A few major primary and secondary sources referred to for preparing this report on healthcare regulatory affairs outsourcing market in North America are company websites, annual reports, financial reports, national government documents, and statistical database, among others. Major companies listed in the report are Arriello Ireland Ltd., IQVIA Inc., PAREXEL INTERNATIONAL CORPORATION, PHARMALEX GMBH, ProductLife Group, ProPharma Group, and Voisin Consulting Life Sciences (VCLS) are among others.
Reasons to buy report
- To understand the North America healthcare regulatory affairs outsourcing market landscape and identify market segments that are most likely to guarantee a strong return
- Stay ahead of the race by comprehending the ever-changing competitive landscape for North America healthcare regulatory affairs outsourcing market
- Efficiently plan M&A and partnership deals in North America healthcare regulatory affairs outsourcing market by identifying market segments with the most promising probable sales
- Helps to take knowledgeable business decisions from perceptive and comprehensive analysis of market performance of various segment form North America healthcare regulatory affairs outsourcing market
- Obtain market revenue forecast for market by various segments from 2021-2028 in North America region.
North America Healthcare Regulatory Affairs Outsourcing Market Segmentation
North America Healthcare Regulatory Affairs Outsourcing Market –By Service Type
- Medical & Scientific Writing
- Pharmacovigilance
- Data Management Services
- Life Cycle Management Services
- eCTD and e-Submissions
- Regulatory and Scientific Strategy development
- Chemistry Manufacturing and Controls (CMC) Services
- Regulatory Labelling
- Regulatory Artwork Services
North America Healthcare Regulatory Affairs Outsourcing Market –By End User
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Devices Companies
- Medical Device Materials & Biomaterials
- Medical Device Biomarkers and In vitro Diagnostics (IVD)
- Medical Device Software (SaMD)
- Medical Device Electromechanics
- Medical Device Substance-based
- Medical Device of Combination Product
North America Healthcare Regulatory Affairs Outsourcing Market -By Country
- US
- Canada
- Mexico
North America Healthcare Regulatory Affairs Outsourcing Market -Company Profiles
- Arriello Ireland Ltd.
- IQVIA Inc.
- PAREXEL INTERNATIONAL CORPORATION
- PHARMALEX GMBH
- ProductLife Group
- ProPharma Group
- Voisin Consulting Life Sciences (VCLS)
TABLE OF CONTENTS
1. Introduction
1.1 Scope of the Study
1.2 The Insight Partners Research Report Guidance
1.3 Market Segmentation
1.3.1 North America Healthcare Regulatory Affairs Outsourcing Market – By Service Type
1.3.2 North America Healthcare Regulatory Affairs Outsourcing Market – By End User
1.3.3 North America Healthcare Regulatory Affairs Outsourcing Market – By Country
2. North America Healthcare Regulatory Affairs Outsourcing Market – Key Takeaways
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. North America Healthcare Regulatory Affairs Outsourcing Market – Market Landscape
4.1 Overview
4.1.1 North America – PEST Analysis
4.2 Expert Opinions
5. North America Healthcare Regulatory Affairs Outsourcing Market– Key Market Dynamics
5.1 Market Drivers
5.1.1 Rising Regulatory Pressure on Healthcare Companies
5.1.2 Escalating Demand for Speedy Approval of New Products
5.2 Market Restraints
5.2.1 Dearth of Skilled Professionals
5.3 Market Opportunities
5.3.1 Advancements in Specialty Therapies, Orphan Drugs, and Personalized Medicines
5.4 Future Trends
5.4.1 Soaring Market Consolidation Activities
5.5 Impact Analysis
6. Healthcare Regulatory Affairs Outsourcing Market – North America Analysis
6.1 North America Healthcare Regulatory Affairs Outsourcing Market Revenue Forecast and Analysis
7. North America Healthcare Regulatory Affairs Outsourcing Market Analysis – By Service Type
7.1 Overview
7.2 Healthcare Regulatory Affairs Outsourcing Market, by Service Type (2021 and 2028)
7.3 Regulatory and Scientific Strategy Development
7.3.1 Overview
7.3.2 Regulatory and Scientific Strategy Development: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.4 Medical and Scientific Writing
7.4.1 Overview
7.4.2 Medical and Scientific Writing: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.5 eCTD and E-Submissions
7.5.1 Overview
7.5.2 eCTD and E-Submissions: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.6 Data Management Services
7.6.1 Overview
7.6.2 Data Management Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.7 Life Cycle Management Services
7.7.1 Overview
7.7.2 Life Cycle Management Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.8 Pharmacovigilance
7.8.1 Overview
7.8.2 Pharmacovigilance : Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.9 Chemistry Manufacturing and Controls (CMC) Services
7.9.1 Overview
7.9.2 Chemistry Manufacturing & Controls ((CMC) Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.10 Regulatory Labelling
7.10.1 Overview
7.10.2 Regulatory Labelling : Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
7.11 Regulatory Artwork Services
7.11.1 Overview
7.11.2 Regulatory Artwork Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
8. North America Healthcare Regulatory Affairs Outsourcing Market Analysis – By End User
8.1 Overview
8.2 Healthcare Regulatory Affairs Outsourcing Market, by End-User (2020 and 2028)
8.3 Pharmaceutical Companies
8.3.1 Overview
8.3.2 Pharmaceutical Companies: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
8.4 Biotechnology Companies
8.4.1 Overview
8.4.2 Biotechnology Companies : Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
8.5 Medical Device Companies
8.5.1 Overview
8.5.2 Medical Device Companies: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
8.5.2.1 Medical Device Software (SaMD) Market
8.5.2.1.1 Overview
8.5.2.1.2 Medical Device Software (SaMD) Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.2 Medical Device Materials & Biomaterials Market
8.5.2.2.1 Overview
8.5.2.2.2 Medical Device Materials & Biomaterials Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.3 Medical Device Biomarkers and In-vitro Diagnostics (IVD) Market
8.5.2.3.1 Overview
8.5.2.3.2 Medical Device Biomarkers and In-vitro Diagnostics (IVD) Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.4 Medical Device Electro mechanics Market
8.5.2.4.1 Overview
8.5.2.4.2 Medical Device Electro mechanics Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.5 Medical Device Substance Based Market
8.5.2.5.1 Overview
8.5.2.5.2 Medical Device Substance Based Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
8.5.2.6 Medical Device of Combination Product (DDC) Market
8.5.2.6.1 Overview
8.5.2.6.2 Medical Device of Combination Product (DDC) Market: Healthcare Regulatory affairs outsourcing Market –Revenue and Forecast to 2028 (US$ Million)
9. North America Healthcare Regulatory Affairs Outsourcing Market – Country Analysis
9.1 Overview
9.1.1 North America: Healthcare Regulatory Affairs Outsourcing Market, by Country, 2021 & 2028 (%)
9.1.1.1 US: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.1.1 US: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.1.2 US Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
9.1.1.1.3 US Healthcare Regulatory Affairs Outsourcing Market, by End User – Revenue and Forecast to 2028 (USD Million)
9.1.1.1.3.1 US Healthcare Regulatory Affairs Outsourcing Market, by Medical Device Companies – Revenue and Forecast to 2028 (USD Million)
9.1.1.2 Canada: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.2.1 Canada: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.2.2 Canada Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
9.1.1.2.3 Canada Healthcare Regulatory Affairs Outsourcing Market, by End User– Revenue and Forecast to 2028 (USD Million)
9.1.1.2.3.1 Canada Healthcare Regulatory Affairs Outsourcing Market, by Medical Device Companies– Revenue and Forecast to 2028 (USD Million)
9.1.1.3 Mexico: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.3.1 Mexico: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
9.1.1.3.2 Mexico Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
9.1.1.3.3 Mexico Healthcare Regulatory Affairs Outsourcing Market, by End User – Revenue and Forecast to 2028 (USD Million)
9.1.1.3.3.1 Mexico Healthcare Regulatory Affairs Outsourcing Market, by Medical Device Companies– Revenue and Forecast to 2028 (USD Million)
10. Impact Of COVID-19 Pandemic on North America Healthcare Regulatory Affairs Outsourcing Market
10.1 Overview
11. Industry Landscape
11.1 Overview
11.2 Organic Developments
11.2.1 Overview
11.3 Inorganic Developments
11.3.1 Overview
12. Company Profiles
12.1 ProPharma Group
12.1.1 Key Facts
12.1.2 Business Description
12.1.3 Products and Services
12.1.4 Financial Overview
12.1.5 SWOT Analysis
12.1.6 Key Developments
12.2 Arriello Ireland Ltd.
12.2.1 Key Facts
12.2.2 Business Description
12.2.3 Products and Services
12.2.4 Financial Overview
12.2.5 SWOT Analysis
12.2.6 Key Developments
12.3 PAREXEL INTERNATIONAL CORPORATION
12.3.1 Key Facts
12.3.2 Business Description
12.3.3 Products and Services
12.3.4 Financial Overview
12.3.5 SWOT Analysis
12.3.6 Key Developments
12.4 IQVIA Inc.
12.4.1 Key Facts
12.4.2 Business Description
12.4.3 Products and Services
12.4.4 Financial Overview
12.4.5 SWOT Analysis
12.4.6 Key Developments
12.5 PHARMALEX GMBH
12.5.1 Key Facts
12.5.2 Business Description
12.5.3 Products and Services
12.5.4 Financial Overview
12.5.5 SWOT Analysis
12.5.6 Key Developments
12.6 ProductLife Group
12.6.1 Key Facts
12.6.2 Business Description
12.6.3 Products and Services
12.6.4 Financial Overview
12.6.5 SWOT Analysis
12.6.6 Key Developments
12.7 Voisin Consulting Life Sciences (VCLS)
12.7.1 Key Facts
12.7.2 Business Description
12.7.3 Products and Services
12.7.4 Financial Overview
12.7.5 SWOT Analysis
12.7.6 Key Developments
13. Appendix
13.1 About The Insight Partners
13.2 Glossary of Terms
LIST OF TABLES
Table 1. North America Healthcare Regulatory Affairs Outsourcing Market Revenue and Forecast to 2028 (US$ Million)
Table 2. US Healthcare Regulatory Affairs Outsourcing Market, by Service Type – Revenue and Forecast to 2028 (USD Million)
Table 3. US Healthcare Regulatory Affairs Outsourcing Market, by End User – Revenue and Forecast to 2028 (USD Million)
Table 4. US Healthcare Regulatory Affairs Outsourcing Devices Market, by Medical Device Companies– Revenue and Forecast to 2028 (USD Million)
Table 5. Canada Healthcare Regulatory Affairs Outsourcing Market, by Service Type -Revenue and Forecast to 2028 (USD Million)
Table 6. Canada Healthcare Regulatory Affairs Outsourcing Market, by End User - Revenue and Forecast to 2028 (USD Million)
Table 7. Canada Healthcare Regulatory Affairs Outsourcing Devices Market, by Medical Device Companies – Revenue and Forecast to 2028 (USD Million)
Table 8. Mexico Healthcare Regulatory Affairs Outsourcing Market, by Service Type -Revenue and Forecast to 2028 (USD Million)
Table 9. Mexico Healthcare Regulatory Affairs Outsourcing Market, by End User – Revenue and Forecast to 2028 (USD Million)
Table 10. Mexico Healthcare Regulatory Affairs Outsourcing Market, by Medical Device Companies – Revenue and Forecast to 2028 (USD Million)
Table 11. Organic Developments in the Healthcare Regulatory Affairs Outsourcing Market
Table 12. Inorganic Developments in the Healthcare Regulatory Affairs Outsourcing Market
Table 13. Glossary of Terms, Healthcare Regulatory Affairs Outsourcing Market
LIST OF FIGURES
Figure 1. North America Healthcare Regulatory Affairs Outsourcing Market Segmentation
Figure 2. North America Healthcare Regulatory Affairs Outsourcing Market Segmentation, By Country
Figure 3. North America Healthcare Regulatory Affairs Outsourcing Market Overview
Figure 4. Medical & Scientific Writing Segment Held the Largest Share of the Service Type Segment in North America Healthcare Regulatory Affairs Outsourcing Market
Figure 5. US to Show Significant Growth During Forecast Period
Figure 6. North America PEST Analysis
Figure 7. North America Healthcare Regulatory Affairs Outsourcing Market Impact Analysis of Driver and Restraints
Figure 8. North America Healthcare Regulatory Affairs Outsourcing Market – Revenue Forecast and Analysis – 2019- 2028
Figure 9. North America Healthcare Regulatory Affairs Outsourcing Market Revenue Share, by Service Type (2020 and 2028)
Figure 10. North America Regulatory and Scientific Strategy Development: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 11. North America Medical and Scientific Writing: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 12. North America eCTD and E-Submissions: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 13. North America Data Management Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 14. North America Life Cycle Management Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 15. North America Pharmacovigilance : Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 16. North America Chemistry Manufacturing & Controls ((CMC) Services: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 17. North America Regulatory Labelling: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 18. North America Regulatory Artwork Services : Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 19. North America Healthcare Regulatory Affairs Outsourcing Market Revenue Share, by End-User (2020 and 2028)
Figure 20. North America Pharmaceutical Companies : Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 21. North America Biotechnology Companies: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 22. North America Medical Device Companies: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 23. North America Medical Device Software (SaMD) Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 24. North America Medical Device Materials & Biomaterials Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 25. North America Medical Device Biomarkers and In-vitro Diagnostics (IVD) Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 26. North America Medical Device Electro mechanics Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 27. North America Medical Device Substance Based Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 28. North America Medical Device of Combination Product (DDC) Market: Healthcare Regulatory affairs outsourcing Market – Revenue and Forecast to 2028 (US$ Million)
Figure 29. North America: Healthcare Regulatory Affairs Outsourcing Market, by Key Country – Revenue (2021) (USD Million)
Figure 30. North America: Healthcare Regulatory Affairs Outsourcing Market, by Country, 2021 & 2028 (%)
Figure 31. US: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
Figure 32. Canada: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
Figure 33. Mexico: Healthcare Regulatory Affairs Outsourcing Market – Revenue and Forecast to 2028 (USD Million)
Figure 34. Impact of COVID-19 Pandemic in North American Country Markets- Arriello Ireland Ltd.
- IQVIA Inc.
- PAREXEL INTERNATIONAL CORPORATION
- PHARMALEX GMBH
- ProductLife Group
- ProPharma Group
- Voisin Consulting Life Sciences (VCLS)
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players and segments in the North America Healthcare Regulatory Affairs Outsourcing market.
- Highlights key business priorities in order to assist companies to realign their business strategies
- The key findings and recommendations highlight crucial progressive industry trends in the North America Healthcare Regulatory Affairs Outsourcing market, thereby allowing players across the value chain to develop effective long-term strategies
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets
- Scrutinize in-depth North America market trends and outlook coupled with the factors driving the Healthcare Regulatory Affairs Outsourcing market, as well as those hindering it
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing and distribution